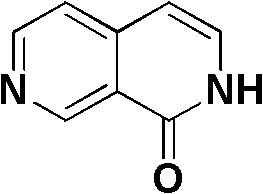
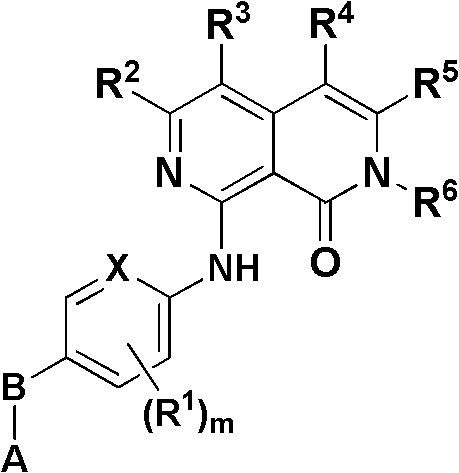
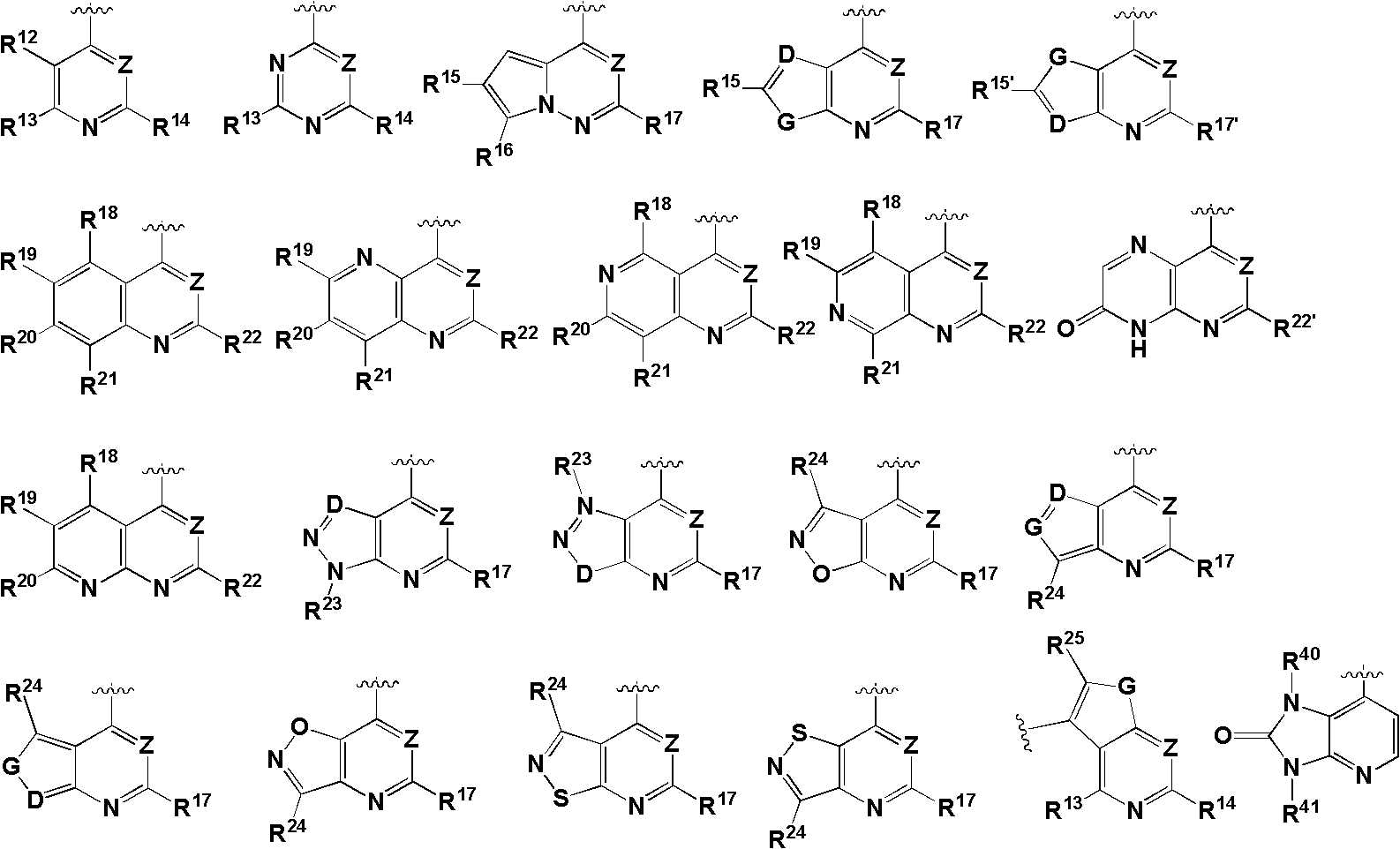
2,7-naphthyridine derivative, and preparation method and application thereof
The technology of a compound, R22, is applied in 2 fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0103] The present invention also relates to a preparation method with general formula (I), characterized in that it comprises,
[0104] a. Base segment (V) and chlorinated heterocycle (VI) in solvent S 1 Alkaline B 1 , Catalyst C 1 , Ligand L 1 In the presence of temperature T 1 Under the conditions, the reaction produces compound (I).
[0105]
[0106] or
[0107] b. Base fragment (V) and chlorinated heterocycle (VI) in solvent S 2 Medium acid A 1 In the presence of temperature T 2 Under the conditions, the reaction produces compound (I).
[0108]
[0109] where A, B, X, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 and m as above.
[0110] In order to prepare the compound described in the general formula (I) of the present invention, the preparation method is characterized in that, in the preparation process, the solvent S 1 Be selected from ether solvents, such as 1,4-dioxane, ethylene glycol dimethyl ether, preferably 1,4-dioxane; the base B 1 Selected from in...
specific Embodiment approach
[0128] The invention discloses a compound and its preparation method, an intermediate of the compound and its preparation method, and the application of the compound as a tyrosine kinase and / or serine-threonine kinase inhibitor. Those skilled in the art can Learn from the content of this article, and appropriately improve the process parameters to achieve. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0129] Below in conjunction with embodiment, fu...
Embodiment 1
[0130] Embodiment 1: the preparation of compound 1
[0131]
[0132] 2-Benzyl-8-((4-((6,7-dimethoxyquinazolin-4-yl)oxy)-3-fluorophenyl)amino)-2,7-naphthyridine-1 (2H)-Kone
[0133] Add 4-((6,7-dimethoxyquinazolin-4-yl)oxy)-3-fluoroaniline (50 mg, 1.0 eq), 2-benzyl-8-chloro-2 , 7-naphthyridin-1(2H)-one (42.9mg, 1.0eq), tris(dibenzylideneacetone)dipalladium (29.2mg, 0.2eq), 1,2-bis(diphenylphosphino)propane (26mg, 0.4eq), sodium tert-butoxide (19.8mg, 1.3eq) and 1,4-dioxane (10mL), pump nitrogen three times, react at 106°C for 4 hours, spot reaction is complete, cool to Room temperature, suction filtration, concentration, crude silica gel column chromatography (PE / EA 6 / 1 to 3.5 / 1) to obtain 2-benzyl-8-((4-((6,7-dimethoxyquinazoline- 4-yl)oxy)-3-fluorophenyl)amino)-2,7-naphthyridin-1(2H)-one (10.8mg), MS: [M+H] + =550.1.
[0134] 1 H-NMR (500M, DMSO-d 6 )δ12.11(b, 1H), 8.57(s, 1H), 8.29-8.30(d, 1H), 8.24-8.27(d, 1H), 7.87-7.89(d, 1H), 7.59(s, 1H) , 7.48-7.50(d, 1H), 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com