Antimicrobial compositions
An antibacterial composition, the technology of the composition, applied in the direction of animal repellants, special packaging objects, plant growth regulators, etc., can solve problems such as reducing MEO
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
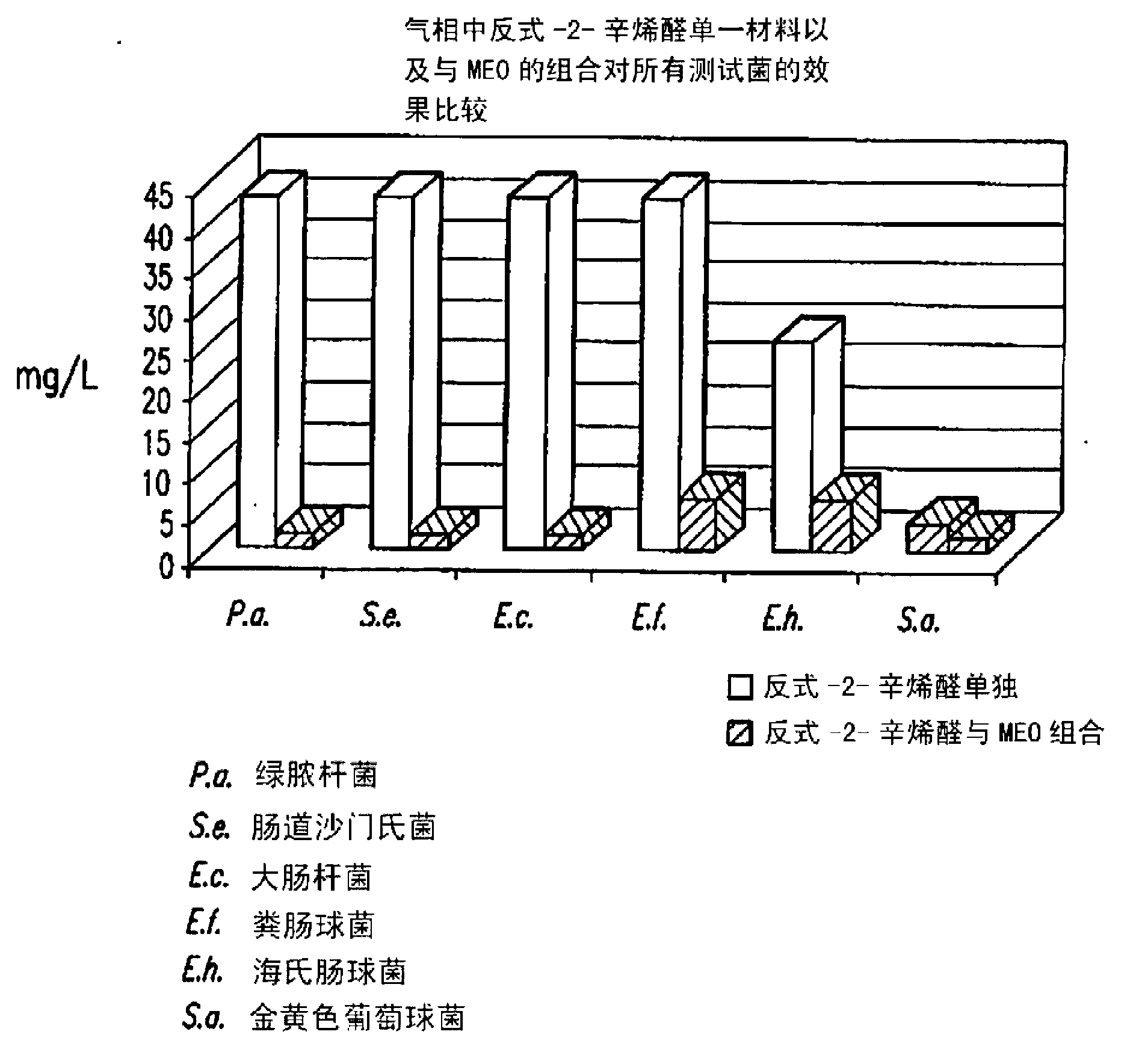
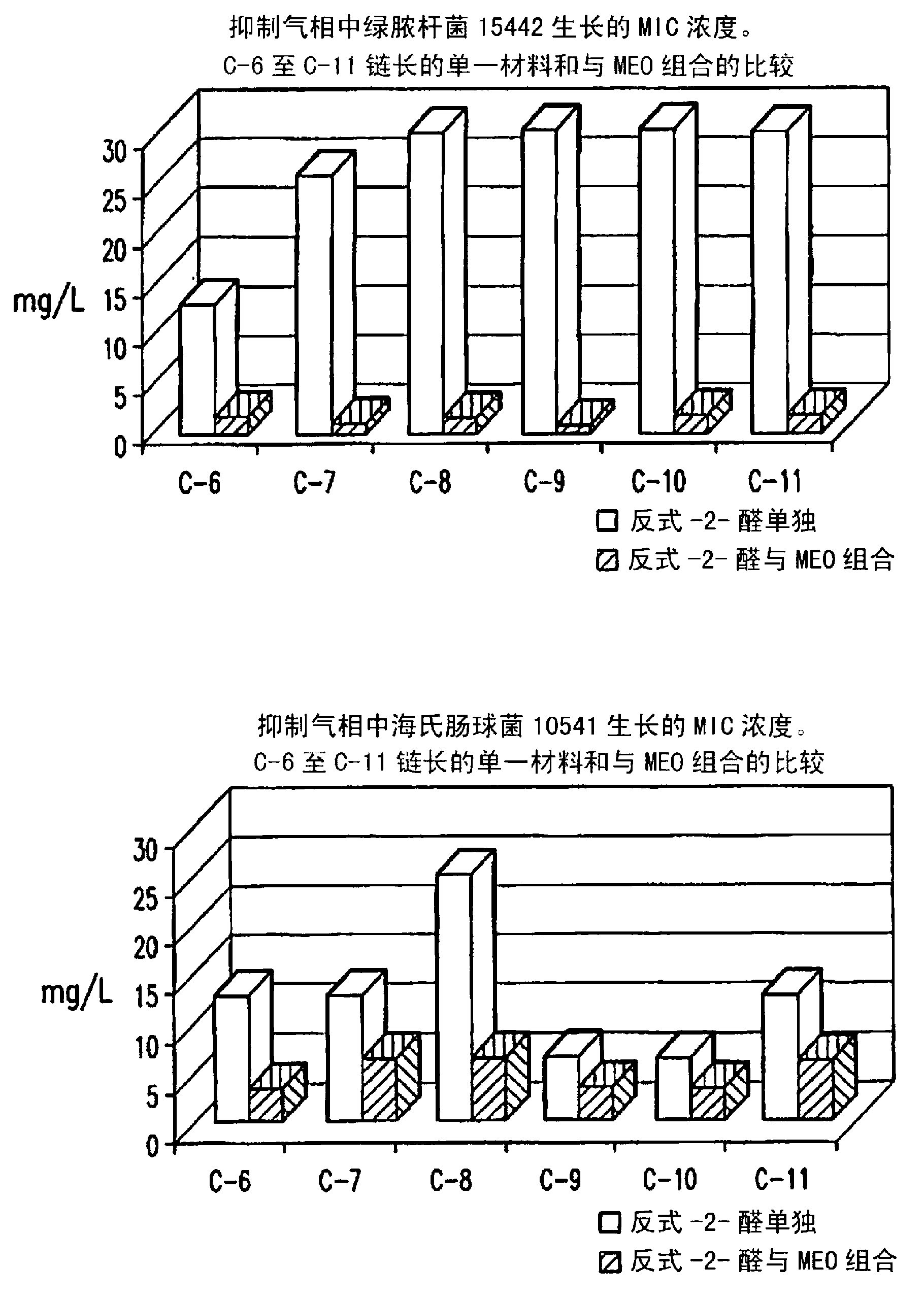
[0058] Brain heart infusion (BHI) agar plates inoculated with E. 7L sealed acrylic box and exposed to trans-2-enal (C 6 -C 11 Carbon chain length) and mustard essential oil (MEO) double combination steam.
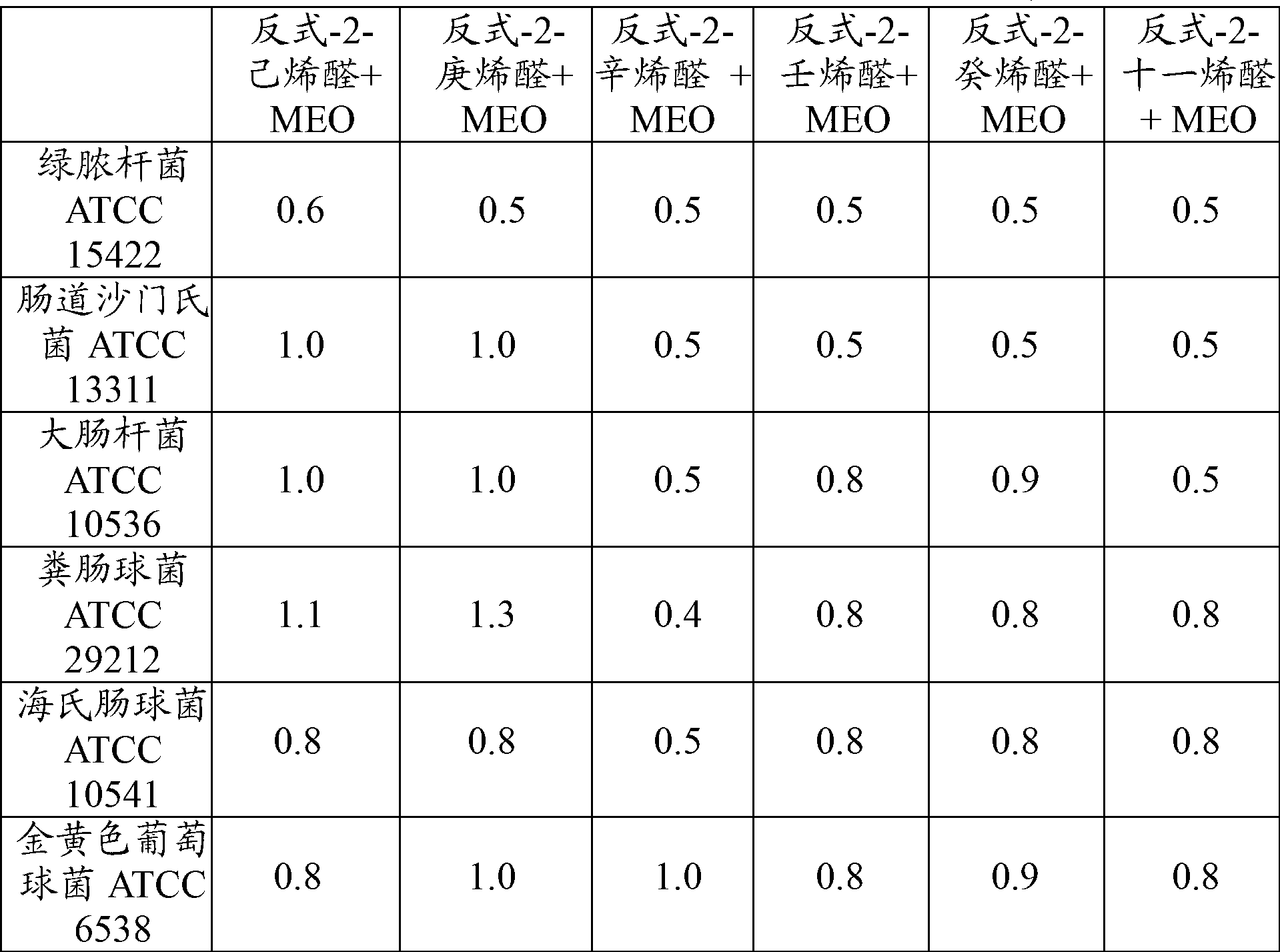
[0059] The trans-2-enal and MEO were weighed net and placed into a glass bottle (the glass bottle was placed in a box between the inoculated agar plates). Concentrations of single substances and double combinations introduced into 7L boxes are expressed as weight per unit volume of the box (mg / L). For each organism, several concentrations of single substances and combinations ranging from 0.5 mg / L to 43 mg / L were tested. Vapor-phase minimum inhibitory concentration (MIC) for "no growth" after 3 days of incubation at room temperature. The partial inhibitory concentration (FIC) was determined according to the following formula to evaluate the antibacterial effect of the combination of trans-2-enal and MEO in the gas phase:
[0060] Combined FIC=(trans-2-enal 组合 MIC / tran...
Embodiment 2
[0081] Brain heart infusion (BHI) agar plates inoculated with E. 7L sealed acrylic box and exposed to vapors of the following compositions. A non-steamed control of the agar plates was established.
[0082] Composition A: trans-2-hexenal at 9.3 mg / L
[0083] Composition B: trans-2-heptenal at 9.3 mg / L
[0084] Composition C: 9.3 mg / L of 2,4-heptadienal
[0085] Composition D: trans-2-hexenal at 7.1 mg / L
[0086] Composition E: 7.1 mg / L of 2,4-heptadienal
[0087] Composition F: 7.1 mg / L of 2,4-heptadienal + citronellol (1:1)
[0088] Composition G: 9.3 mg / L of citronellal + citral DMA (1:1)
[0089] Composition H: 9.3 mg / L benzaldehyde + citral DMA (1:1)
[0090] Composition I: 9.3 mg / L furfural + citronellol (1:1)
[0091] Composition J: citral+citronellal (1:1) at 7.1 mg / L
[0092] Composition K: 7.1 mg / L of trans-2-hexenal + citronellol (1:1)
[0093] and get the following result:
[0094] Table 7: Gas phase activity against bacteria
[0095]
[0096] Label: ...
Embodiment 3
[0101] A detergent base was obtained based on a commercially available gel detergent product and used as a positive control. In each test, a positive control containing 0.2% by weight of trans-2-nonenal, trans-2-decenal, trans-2-undecenal, trans-2-dodecanal Aromatic composition of aldehydes of alkenal and trans-2-tridecenal.
[0102] The activity log reduction of these compositions against Enterococcus hirae ATCC 10541 and Staphylococcus aureus ATCC 6538 in solution was determined. Dilute with 116 grams of water per 1 gram of product and stir with a stir bar for about 10 minutes. Bacteria were added in one aliquot, mixed on a vortex mixer and placed in a 50°C water bath. After 1 hour, samples were diluted in D / E neutralizing broth (Dey and Engley) and spread on solid medium. Surviving bacteria were counted after 1 day of incubation at 37°C. The results are shown in Table 8 below.
[0103] Table 8: Log reduction in activity of Enterococcus hirae ATCC 10541 and Staphylococc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com