Voglibose tablet and preparation method thereof
A technology for voglibose and sugar tablets, applied in the field of voglibose tablets and their preparation, can solve the problems of inability to meet huge demand, few manufacturers, etc., and achieves reduction of labor intensity of workers, low production cost, and convenience Easy to operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
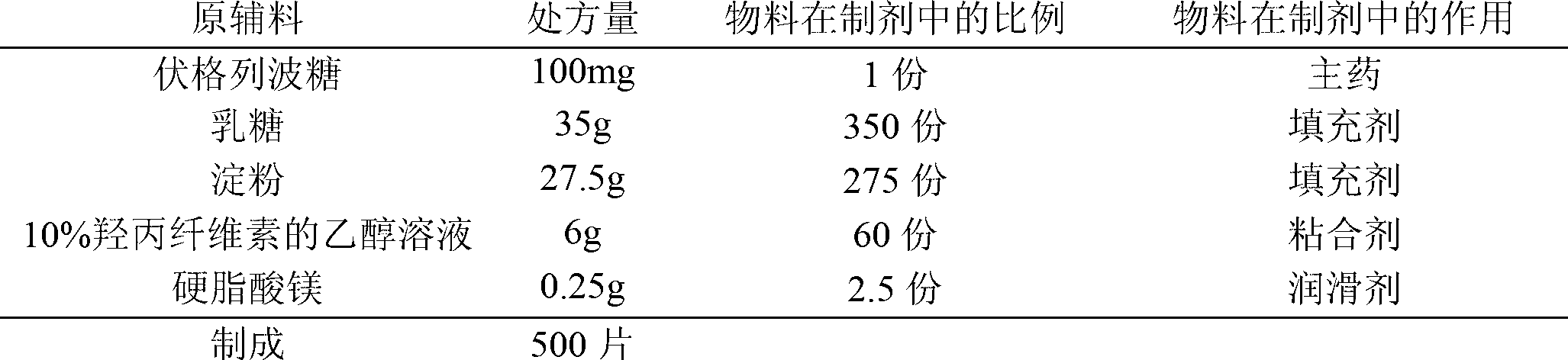
[0024] prescription:
[0025]
[0026] Preparation:
[0027] (1) Pass the voglibose through a 80-mesh sieve, and pass the filler and lubricant through a 100-mesh sieve for later use.
[0028] (2) Weigh the prescribed amount of voglibose and the filling agent and mix them uniformly by incremental method. Mix the prescribed amount of voglibose with 1% (0.275g) of the prescribed amount of the filling agent (starch) first, Then add 4% (1.1g) of the prescription amount of filler (starch) and mix evenly, then add 10% (2.75g) of the prescription amount of filler (starch) and mix evenly, then add 15% of the prescription amount of filler (starch) (4.125g) mixed well and finally the blended mixture was mixed well with the remaining filler (remaining starch (19.525g) and 100% lactose).
[0029] (3) Add an appropriate amount of binder to make soft materials, granulate with a 20-mesh sieve, dry, pass through a 18-mesh sieve for granulation and set aside.
[0030] (4) Add the prescrib...
Embodiment 2
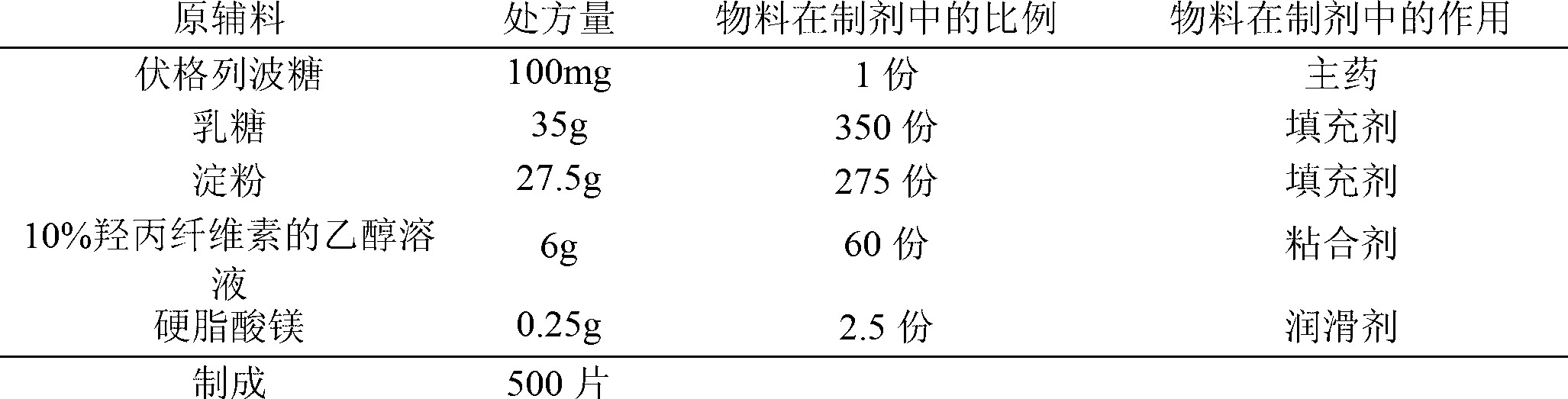
[0032] prescription:
[0033]
[0034] Preparation:
[0035] (1) Pass the voglibose through a 80-mesh sieve, and pass the filler and lubricant through a 100-mesh sieve for later use.
[0036] (2) Weigh the prescribed amount of voglibose and fillers and mix them evenly by equal volume addition method.
[0037] (3) Add an appropriate amount of binder to make soft materials, granulate with a 20-mesh sieve, dry, pass through a 18-mesh sieve for granulation and set aside.
[0038] (4) Add the prescribed amount of lubricant to the granules, mix for 15 minutes, and press into tablets to obtain the finished product.
Embodiment 3
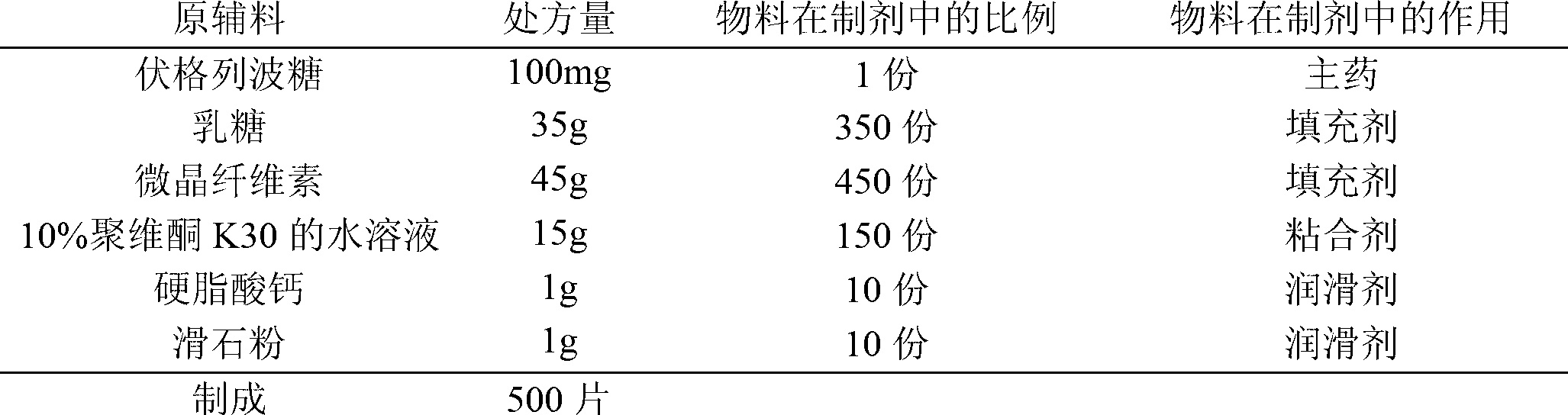
[0040] prescription:
[0041]
[0042] Preparation:
[0043] (1) Pass the voglibose through a 80-mesh sieve, and pass the filler and lubricant through a 100-mesh sieve for later use.
[0044] (2) Weigh the prescribed amount of voglibose and fillers and mix them evenly by equal volume addition method.
[0045] (3) Add an appropriate amount of binder to make soft materials, granulate with a 20-mesh sieve, dry, pass through a 18-mesh sieve for granulation and set aside.
[0046] (4) Add the prescribed amount of lubricant to the granules, mix for 15 minutes, and press into tablets to obtain the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com