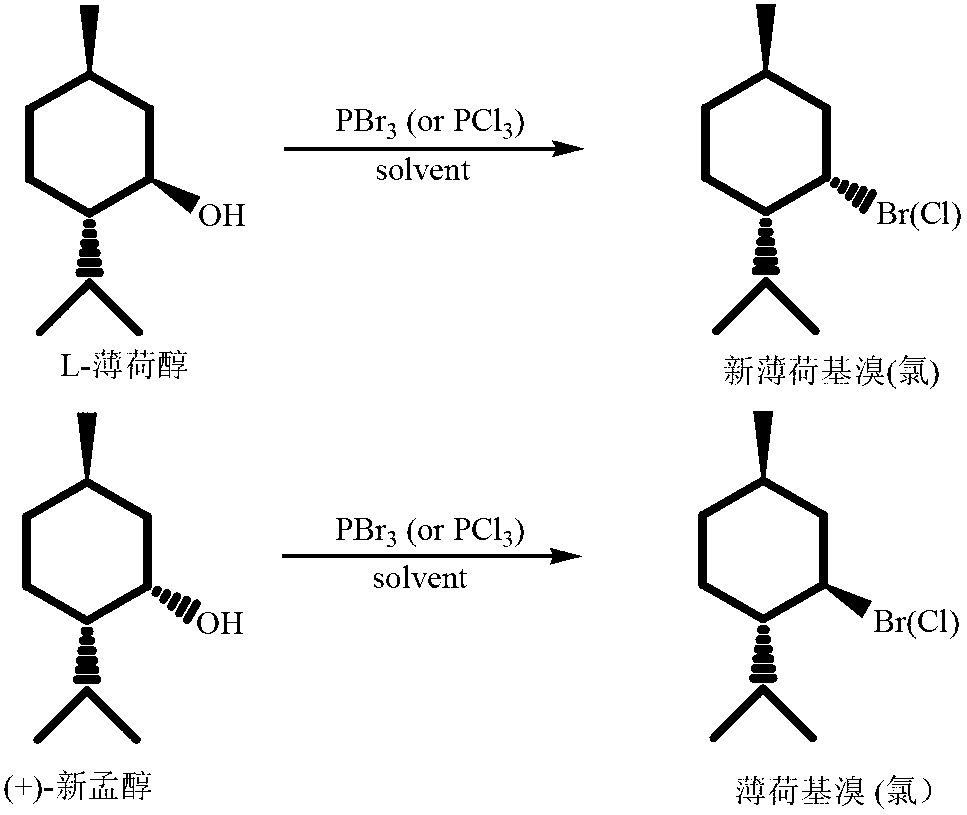
High-stereoselectivity method for synthesizing menthyl halide
A technology of stereoselectivity and synthetic method, which is applied to the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc., can solve the problems of harsh reaction conditions, complicated methods, and no de value given, and achieve high selectivity, The effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Under nitrogen protection, add 15mmol of L-menthol and 20mL of anhydrous ether to a 50mL three-necked flask, slowly add 5mmol of phosphorus tribromide dropwise to the three-necked flask under ice bath, control the rate of addition, so that the reaction temperature is not higher than 5 ℃, after the dropwise addition, react at 0℃ for 2h, then raise the temperature to 25℃ for reaction, TLC detects the reaction end point, add saturated aqueous sodium carbonate solution, separate the organic layer, extract the aqueous layer with ether three times, and wash three times with water, and anhydrous MgS0 4 Drying, separation by column chromatography (eluent volume ratio n-hexane:ethyl acetate=20:1) yielded 2.81 g of the target product, neomenthyl bromide, with a yield of 85.4%. 1 HNMR detection de>99%.
[0023] 1 H NMR (CDCl 3 , 400MHz) δ: 4.67 (m, 1H, CBrH), 2.14-2.19 (m, 1H), 1.72-1.77 (m, 2H), 1.32-1.54 (m, 5H), 0.74-0.67 (m, 10H).
Embodiment 2
[0025] Under nitrogen protection, add 15mmol of L-menthol and 20mL of anhydrous ether to a 50mL three-necked flask, slowly add 10mmol of phosphorus tribromide to the three-necked flask under ice bath, and control the rate of addition so that the reaction temperature is not higher than 5 ℃, after the dropwise addition, react at 0℃ for 2h, then raise the temperature to 25℃ for reaction, TLC detects the reaction end point, add saturated aqueous sodium carbonate solution, separate the organic layer, extract the aqueous layer with ether three times, and wash three times with water, and anhydrous MgS0 4 Drying, separation by column chromatography (eluent volume ratio n-hexane:ethyl acetate=20:1) yielded 2.98g of the target product, neomenthyl bromide, with a yield of 90.6%. 1 HNMR detection de>99%.
[0026] 1 H NMR (CDCl 3 , 400MHz) δ: 4.67 (m, 1H, CBrH), 2.14-2.19 (m, 1H), 1.72-1.77 (m, 2H), 1.32-1.54 (m, 5H), 0.74-0.67 (m, 10H).
Embodiment 3
[0028] Under the protection of nitrogen, add 15mmol of L-menthol and 20mL of anhydrous ether to a 50mL three-necked flask, slowly add 15mmol of phosphorus tribromide to the three-necked flask under ice bath, and control the rate of addition so that the reaction temperature is not higher than 5 ℃, after the dropwise addition, react at 0℃ for 2h, then raise the temperature to 25℃ for reaction, TLC detects the reaction end point, add saturated aqueous sodium carbonate solution, separate the organic layer, extract the aqueous layer with ether three times, and wash three times with water, and anhydrous MgS0 4 Drying and separation by column chromatography (eluent volume ratio n-hexane:ethyl acetate=20:1) yielded 3.01 g of the target product, neomenthyl bromide, with a yield of 91.5%. 1 HNMR detection de>99%.
[0029] 1 HNMR (CDCl 3 , 400MHz) δ: 4.67 (m, 1H, CBrH), 2.14-2.19 (m, 1H), 1.72-1.77 (m, 2H), 1.32-1.54 (m, 5H), 0.74-0.67 (m, 10H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com