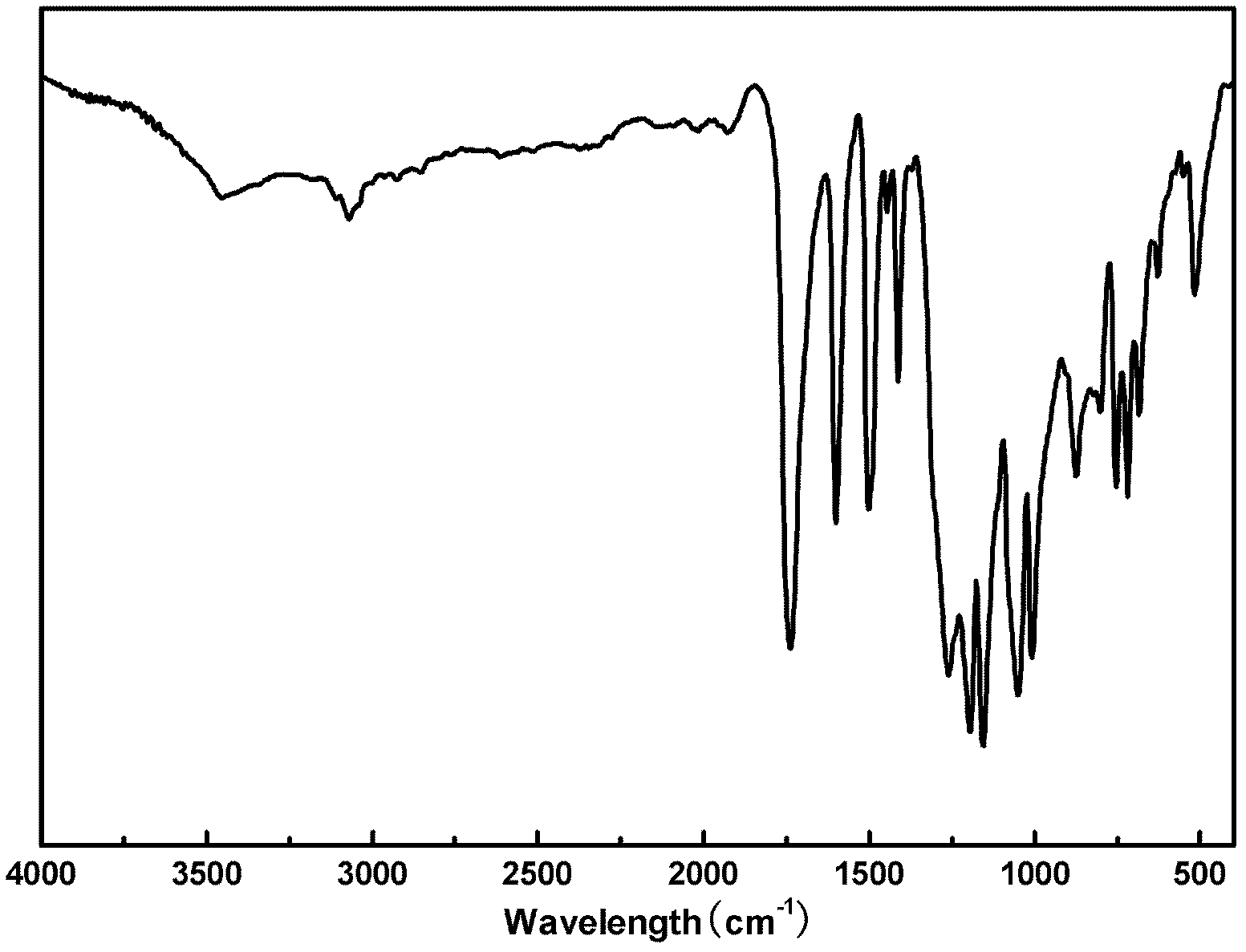
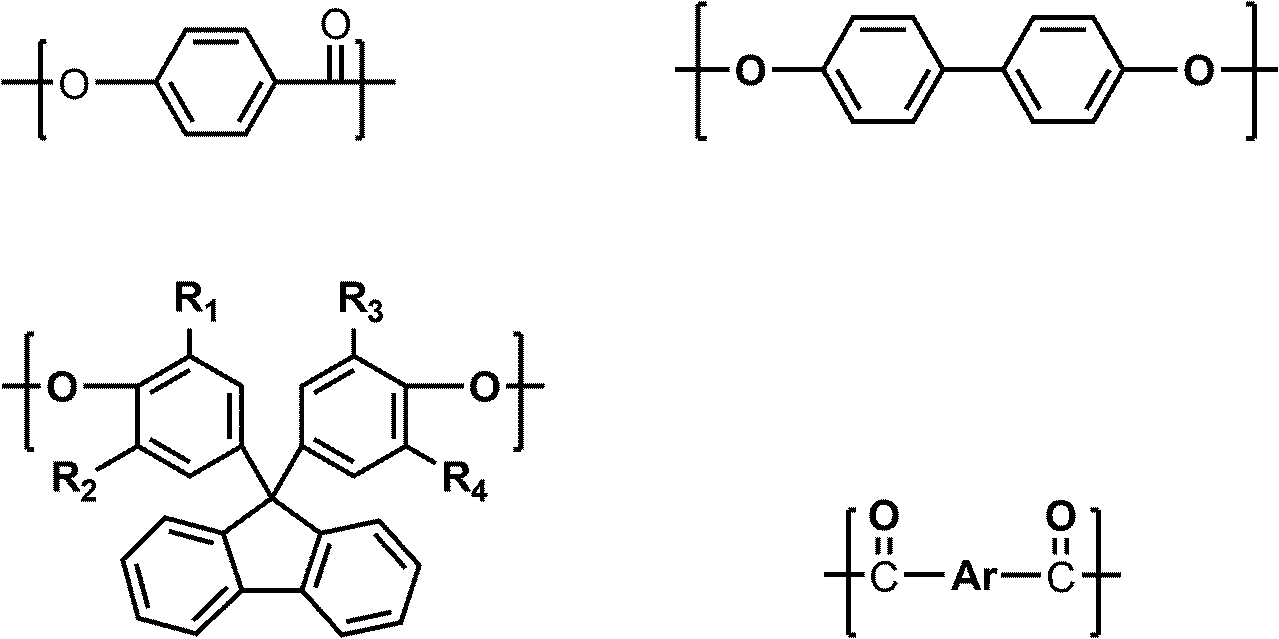
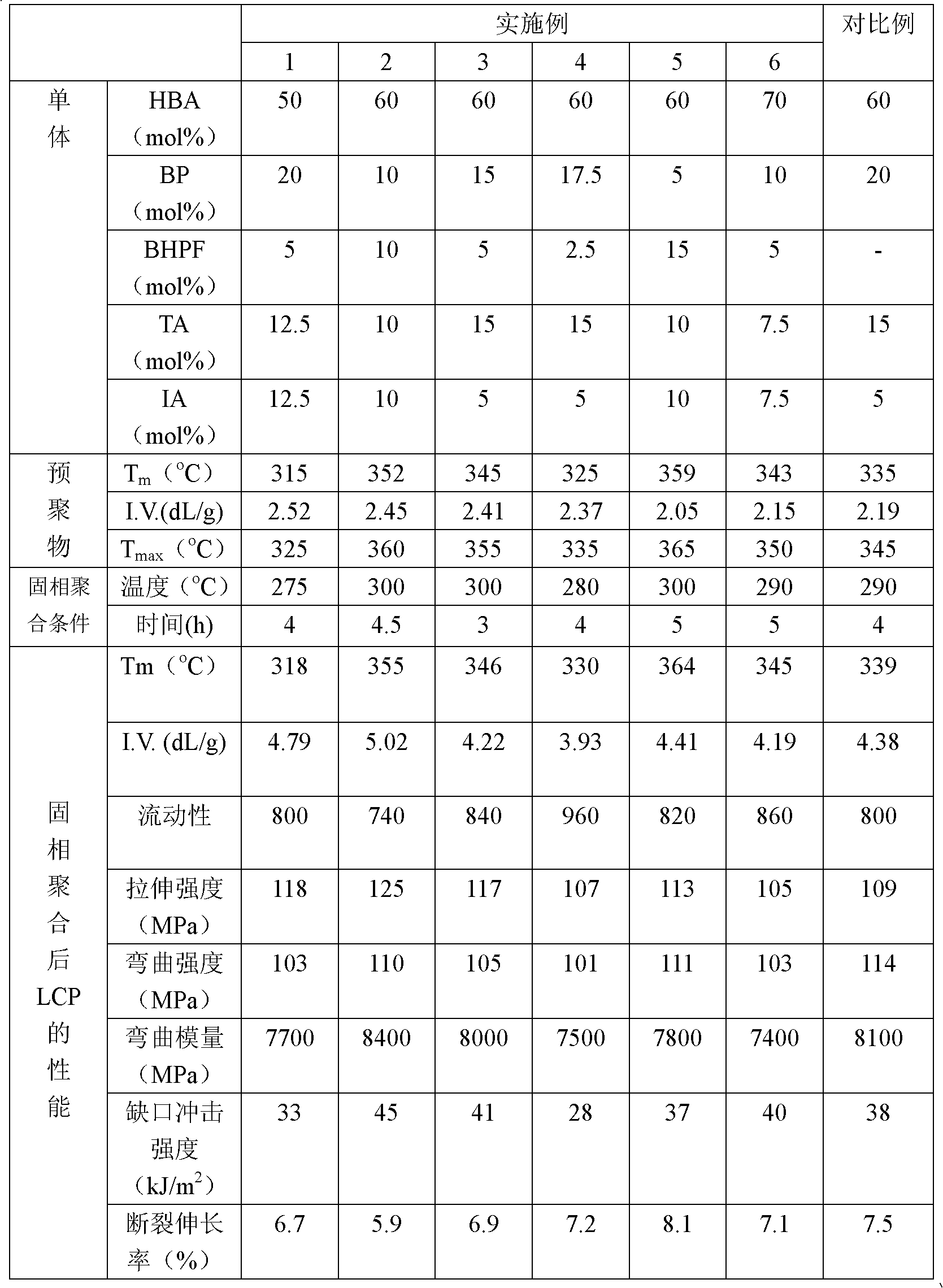
Liquid crystal polyester and its preparation method and use
A technology of liquid crystal polyester and polycondensation reaction, which is applied in the direction of liquid crystal materials, chemical instruments and methods, etc., can solve the problems of synthetic liquid crystal polyester, etc., and achieve excellent melt processing performance, good fluidity, excellent melt processing performance and mechanical properties performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Synthesis of prepolymer
[0045] with N 2 In the gas inlet, condenser and the reactor with a stirrer with a stirring power display, the raw material monomer, catalyst and acylating agent are added in sequence after nitrogen replacement three times,
[0046] Raw material monomer: p-hydroxybenzoic acid (HBA) 690.6g (5mol);
[0047] Biphenol (BP) 372.5g (2mol);
[0048] 9,9-bis(4-hydroxyphenyl)fluorene (BHPF) 175.2g (0.5mol);
[0049] Terephthalic acid (TA) 207.7g (1.25mol);
[0050] Isophthalic acid (IA) 207.7g (1.25mol);
[0051]Acylating agent: acetic anhydride 1235.3g (12.1mol);
[0052] Catalyst: 0.413 g of magnesium acetate.
[0053] After loading the raw materials, the temperature of the reaction system was raised to 145° C. for reflux reaction for 3 hours; then the temperature was raised to 305° C. within 150 minutes, and the reaction was kept at this temperature for 30 minutes. Then raise the temperature to 325°C and react for 30 minutes, then replace t...
Embodiment 2
[0057] (1) Synthesis of prepolymer
[0058] Add 828.8g (6mol) of HBA, 186.3g (1mol) of BP, 350.4g (1mol) of BHPF, 166.1g (1mol) of TA, 166.1g (1mol) of IA, 1235.3g (12.1mol) of acetic anhydride and 0.425g of magnesium acetate Into the reactor, raise the temperature of the reaction system to 145°C for reflux reaction for 4 hours; then raise the temperature to 305°C within 150 minutes, and keep the reaction at this temperature for 30 minutes. Then raise the temperature to 360°C and react for 3 hours, then replace the condenser with a vacuum device, and slowly increase the vacuum degree to polymerize to a predetermined level of torque. Then replace with nitrogen. Take out the prepolymer and pulverize it with a universal pulverizer to obtain a granular prepolymer.
[0059] (2) Solid phase polymerization
[0060] The prepolymer was vacuum-dried at 120°C for 2 hours, then added to a device capable of drawing a high vacuum, heated to 300°C, and reacted for 4.5 hours under the cond...
Embodiment 3
[0062] (1) Synthesis of prepolymer
[0063] HBA 828.8g (6mol), BP 279.5g (1.5mol), BHPF 175.2g (0.5mol), TA 249.2g (1.5mol), IA 83.1g (0.5mol), acetic anhydride 1235.3g (12.1mol) and acetic acid Add 0.404 g of magnesium into the reactor, raise the temperature of the reaction system to 140° C. for reflux reaction for 6 hours; then raise the temperature to 305° C. within 150 minutes, and keep the reaction at this temperature for 30 minutes. Then raise the temperature to 355°C and react for 2.5 hours, then replace the condenser with a vacuum device, and slowly increase the vacuum degree to polymerize to a predetermined level of torque. Then replace with nitrogen. Take out the prepolymer and pulverize it with a universal pulverizer to obtain a granular prepolymer.
[0064] (2) Solid phase polymerization
[0065] The prepolymer was vacuum-dried at 120°C for 2 hours, then added to a device capable of drawing a high vacuum, heated to 300°C, and reacted at 80Pa for 3 hours to obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com