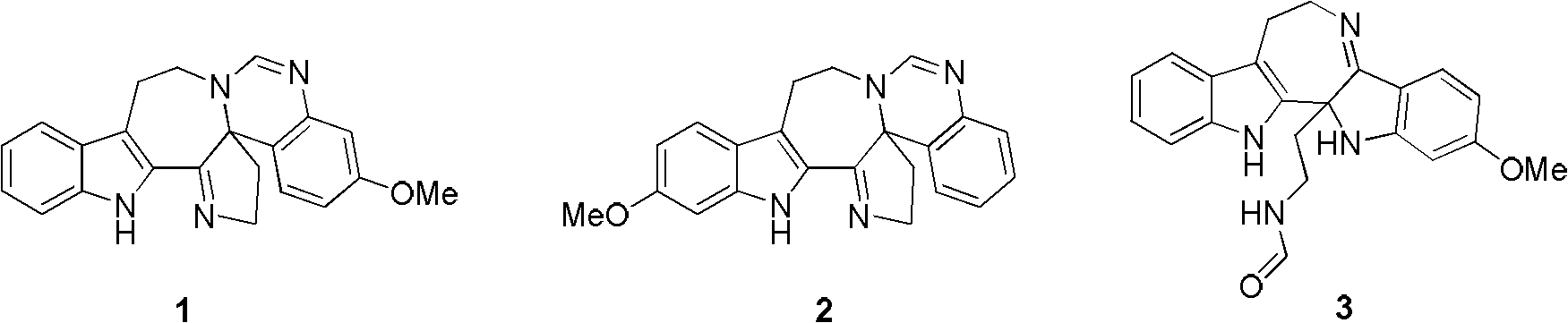
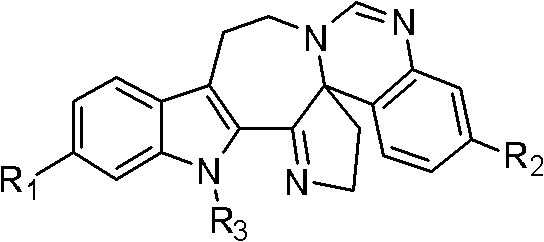
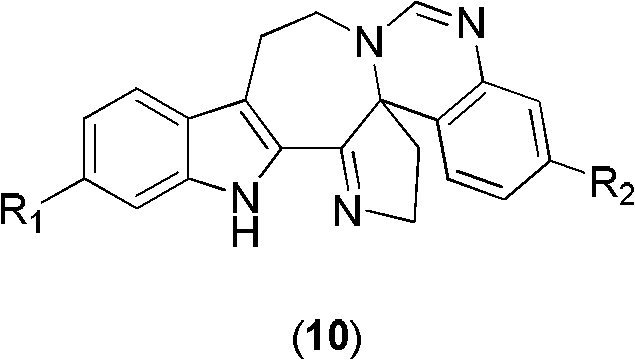
Six-ring indole alkaloid and preparation method thereof
A compound and C1-C12 technology, applied in the field of hexacyclic indole alkaloids and their preparation, can solve the problems of single product, destruction of ecology, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1. Preparation of six-ring indole alkaloids of formula VII (as shown in formula (33))
[0073]
[0074] 1) Dissolve 1.82 g of the compound represented by formula (28) (corresponding to the compound represented by formula II) in 200 mL of ether, and slowly add 6.26 mL of a strong basic compound of tert-butyl lithium in n-pentane solution in an inert atmosphere at -40°C (In this system, the molar ratio of the compound represented by formula (28) to tert-butyl lithium is 1.0:3.0), the reaction is stirred at -40°C for 0.5 hours and then moved to room temperature and 25°C for 1 hour; add to the system 0.25mL of secondary water, followed by 0.82g of compound b paraformaldehyde (the compound represented by formula (28) in the system, the molar ratio of secondary water to paraformaldehyde with a molecular weight of 303.0g / mol is 1.0:5.0: 10.0), the reaction was stirred at room temperature and 25°C for 2.5 hours. The reaction was quenched by adding water twice, extracted wi...
Embodiment 2
[0079] Example 2. Preparation of compound represented by formula VIII, formula (34)
[0080]
[0081] 1) 0.06 g of the compound represented by formula (33) prepared in Example 1 was dissolved in 2 mL of tetrahydrofuran, and added to 30 mL of liquid ammonia, and the reaction was carried out in a nitrogen atmosphere at -76°C. 0.055 g of sodium (the molar ratio of the compound represented by formula (33) to sodium in the system is 1.0:23.5) was added to the above system part by part until a blue solution was formed, and the reaction was stirred for 15 minutes. The reaction was quenched with solid ammonium chloride, heated to room temperature to volatilize ammonia gas, added twice with water, extracted with dichloromethane (4x10mL), and the organic phase was washed with MgSO 4 It is dried, filtered, concentrated, and purified by flash chromatography to obtain 0.031 g of the compound represented by formula (34) (that is, the compound represented by formula VIII) (yield 68%). Brown sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com