High-throughput quantitative detection method of rsv protein content and detection kit thereof
A technology for quantitative detection and protein content, which is applied in the field of quantitative detection of virus titers, can solve the problems of inappropriate compound screening and low sensitivity, and achieve the effects of reducing human factors and consumable use, high sensitivity, and improving throughput
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Non-clinical sample RSV (respiratory syncytial virus) quantitative, high-throughput ELISA detection
[0039] 1. Cell culture stage
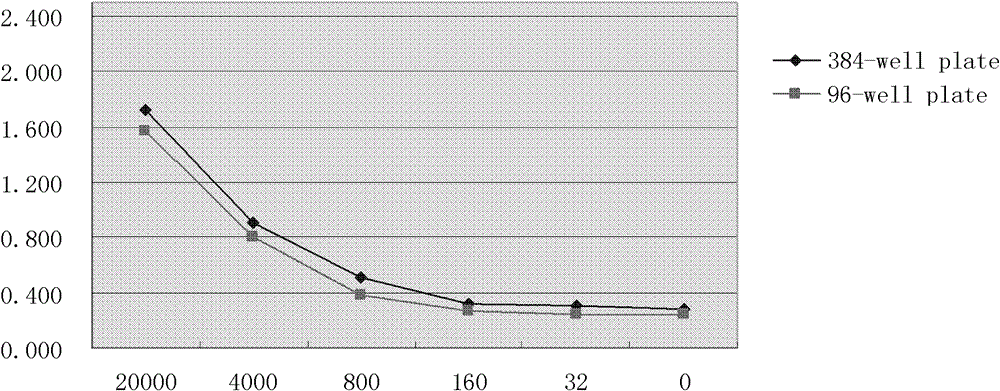
[0040] (1) Hep2 cells (ATCC-CC123) were inoculated into 384-well cell culture plates (Greiner-781080, USA) with 3000 cells in a volume of 30 μl, and placed in a cell culture incubator (37 ° C, 5% CO 2 ) for 16 hours;
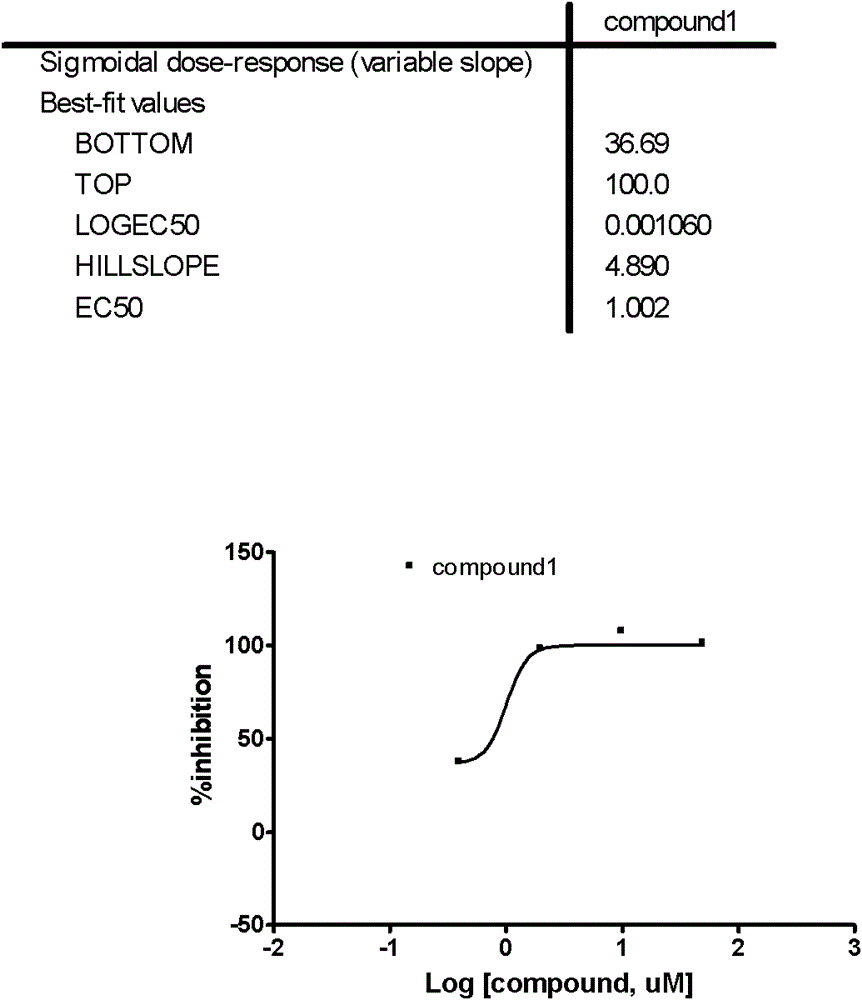
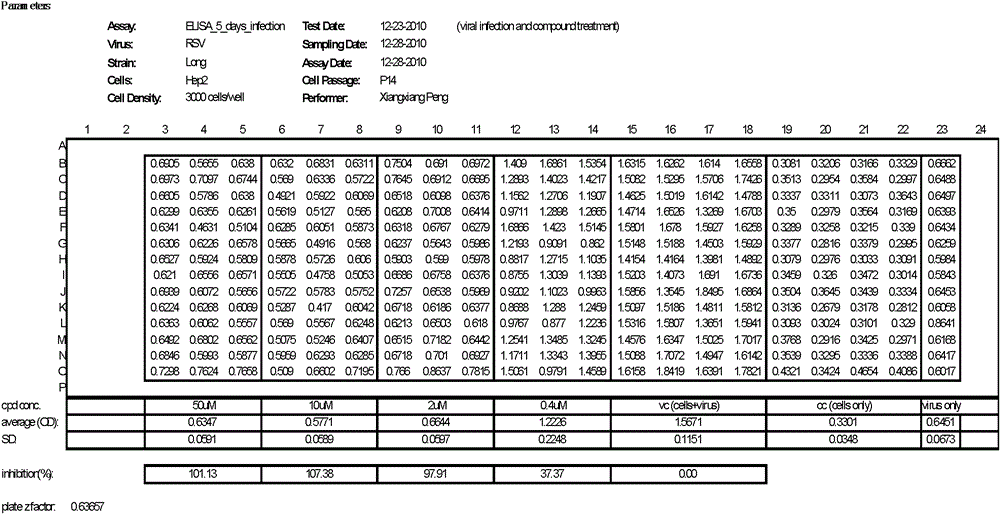
[0041] (2) Dilute non-clinical samples (i.e. compound 1, sourced from customers), according to the final concentration of 50μM, 10μM, 2μM, 0.4μM, respectively take 300nL, 60nL, 12.5nL, 2.5nL from the mother solution (10mM), add to the inoculation 384-well culture plate with cells;
[0042] (3) Dilute RSV (respiratory syncytial virus) (ATCC-VR26), the amount added is: 100 times TCID 50 Virus diluent, the volume is 30 μl;
[0043] (4) After culturing the above 384-well culture plate for 96 hours in a cell culture incubator, the cell culture supernatant was taken for ELISA detection.
[0044] 2. ELISA screening c...
Embodiment 2
[0063] Example 2 Non-clinical sample RSV (respiratory syncytial virus) quantitative, high-throughput ELISA detection
[0064] 1. Cell culture stage
[0065] (1) Hep2 cells (ATCC-CC123) were inoculated into 384-well cell culture plates (Greiner-781080, USA) with 3000 cells in a volume of 30 μl, and placed in a cell culture incubator (37 ° C, 5% CO 2 ) for 16 hours;
[0066] (2) Dilute the non-clinical sample (i.e. compound 1, the compound is from the customer), according to the final concentration of 50μM, 10μM, 2μM, 0.4μM, respectively take 300nL, 60nL, 12.5nL, 2.5nL from the mother solution (10mM), add to Inoculate a 384-well culture plate with cells;
[0067] (3) Dilute RSV (respiratory syncytial virus) (ATCC-VR26), the amount added is: 150 times TCID 50 Virus diluent, the volume is 30 μl;
[0068] (4) After culturing the above 384-well culture plate for 96 hours in a cell culture incubator, the cell culture supernatant was taken for ELISA detection.
[0069] 2. ELISA s...
Embodiment 3
[0088] Example 3 Non-clinical sample RSV (respiratory syncytial virus) quantitative, high-throughput ELISA detection
[0089] 1. Cell culture stage
[0090] (1) Hep2 cells (ATCC-CC123) were inoculated into 384-well cell culture plates (Greiner-781080, USA) with 3000 cells in a volume of 30 μl, and placed in a cell culture incubator (37 ° C, 5% CO 2 ) for 16 hours;
[0091] (2) Dilute the non-clinical sample (i.e. compound 1, the compound is from the customer), according to the final concentration of 50μM, 10μM, 2μM, 0.4μM, respectively take 300nL, 60nL, 12.5nL, 2.5nL from the mother solution (10mM), add to Inoculate a 384-well culture plate with cells;
[0092] (3) Dilute RSV (respiratory syncytial virus) (ATCC-VR26), the amount added is: 150 times TCID 50 Virus diluent, the volume is 30 μl;
[0093] (4) After culturing the above 384-well culture plate for 96 hours in a cell culture incubator, the cell culture supernatant was taken for ELISA detection.
[0094] 2. ELISA s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com