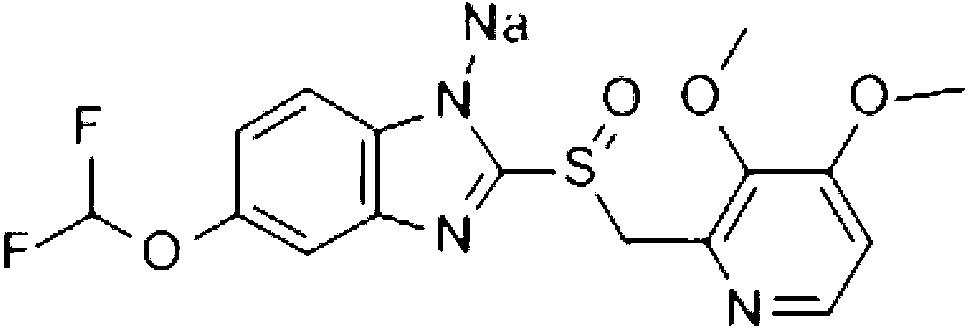
Novel pantoprazole sodium compound and pharmaceutical composition thereof
A kind of technology of pantoprazole sodium and compound, applied in the field of novel pantoprazole sodium compound and pharmaceutical composition thereof, can solve the problems of chemical structure destructive change, discoloration, low purity of pantoprazole sodium, etc., and achieve improvement The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A kind of pharmaceutical composition of pantoprazole sodium compound, described pharmaceutical composition is injection, and described injection is prepared by the following steps:
[0019] (1) Take 400ml of water for injection and put it in a container, then add 500mg of disodium edetate, 5mg of sodium tartrate and 520mg of mannitol, dissolve and add activated carbon, filter to form solution A;
[0020] (2) Take 100 ml of water for injection and place it in another container, slowly add 450 mg of pantoprazole sodium, and stir until the pantoprazole sodium is completely dissolved to form solution B;
[0021] (3) Add solution B to solution A, add water for injection to 1000ml, add activated carbon again, stir and filter to form solution C;
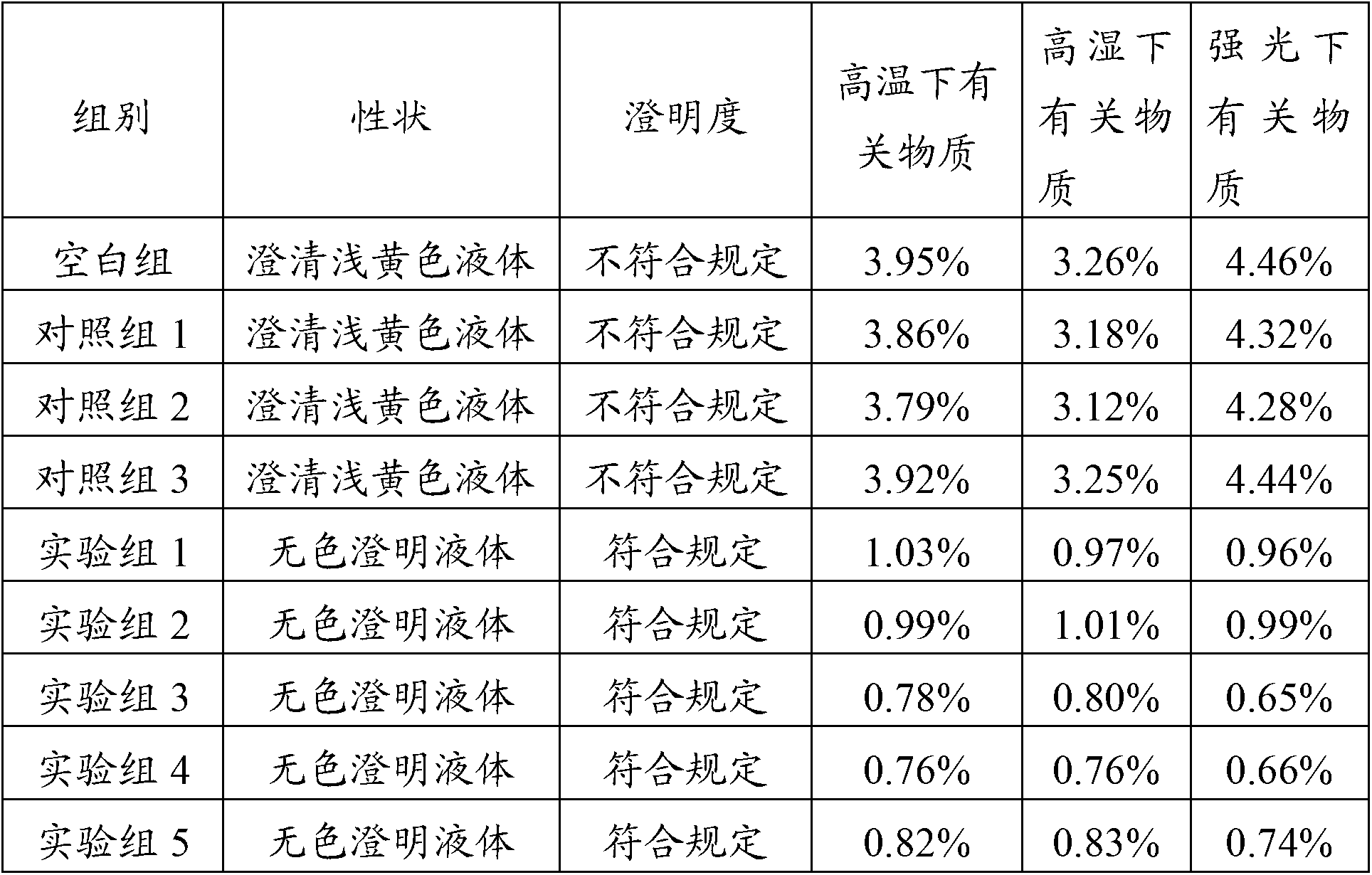
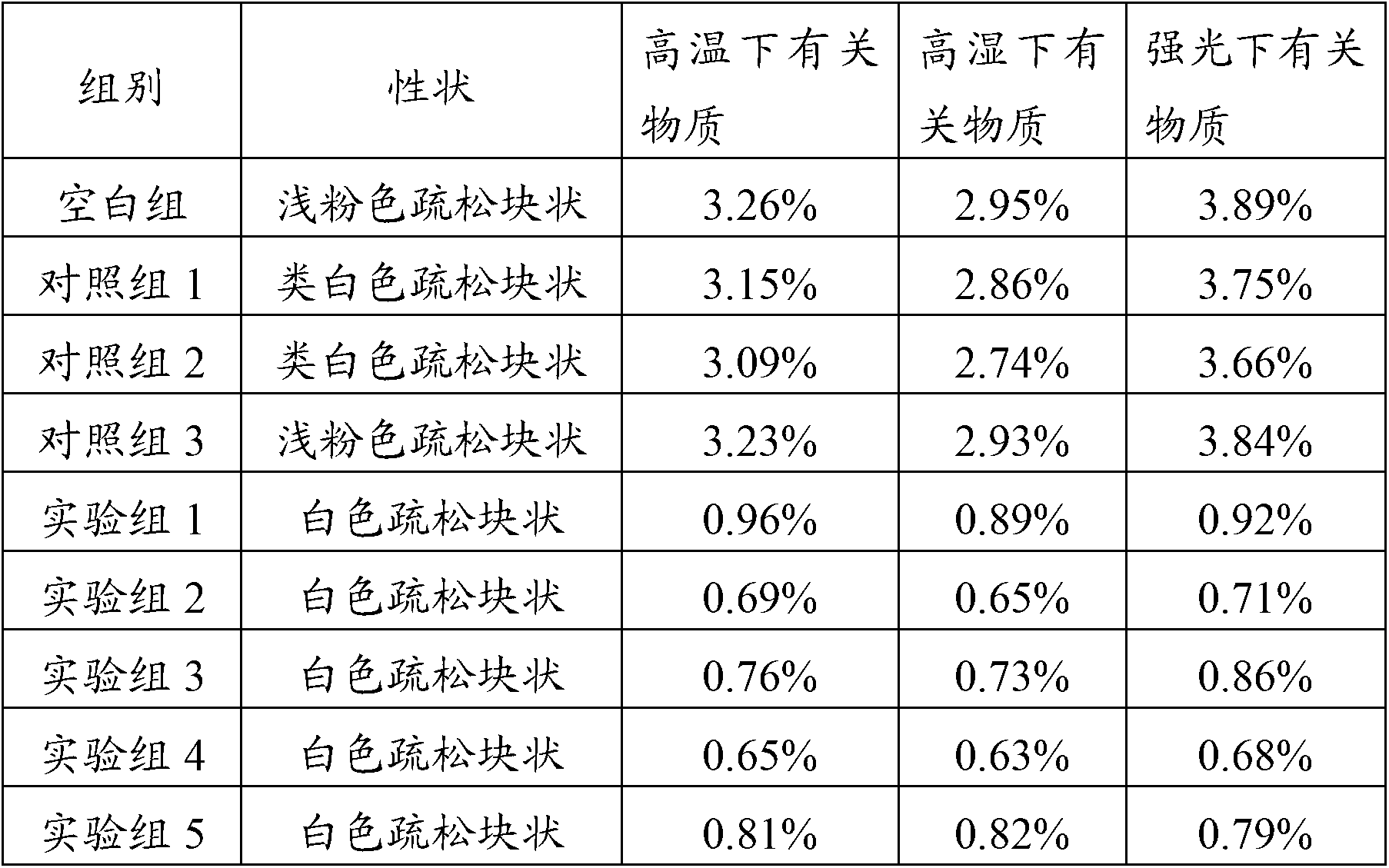
[0022] (4) Detect the content of pantoprazole sodium in the solution C, and the qualified solution can be packaged after fine filtration, potting and sterilization to form a pharmaceutical composition injection of pantoprazole sodium...
Embodiment 2
[0024] A kind of pharmaceutical composition of pantoprazole sodium compound, described pharmaceutical composition is injection, and described injection is prepared by the following steps:
[0025] (1) Take 200ml of water for injection and place it in a container, then add 30mg of disodium edetate, 30mg of sodium tartrate and 40mg of mannitol, dissolve and add activated carbon, filter to form solution A;
[0026] (2) Take 200 ml of water for injection and place it in another container, slowly add 800 mg of pantoprazole sodium, and stir until the pantoprazole sodium is completely dissolved to form solution B;
[0027] (3) Add solution B to solution A, add water for injection to 1000ml, add activated carbon again, stir and filter to form solution C;
[0028] (4) Detect the content of pantoprazole sodium in the solution C, and the qualified solution can be packaged after fine filtration, potting and sterilization to form a pharmaceutical composition injection of pantoprazole sodiu...
Embodiment 3
[0030] A kind of pharmaceutical composition of pantoprazole sodium compound, described pharmaceutical composition is injection, and described injection is prepared by the following steps:
[0031] (1) Take 200ml of water for injection and put it in a container, then add 120mg of disodium edetate, 20mg of sodium tartrate and 30mg of mannitol, dissolve and add activated carbon, filter to form solution A;
[0032] (2) Take 150 ml of water for injection and place it in another container, slowly add 600 mg of pantoprazole sodium, and stir until the pantoprazole sodium is completely dissolved to form solution B;
[0033] (3) Add solution B to solution A, add water for injection to 1000ml, add activated carbon again, stir and filter to form solution C;
[0034] (4) Detect the content of pantoprazole sodium in the solution C, and the qualified solution can be packaged after fine filtration, potting and sterilization to form a pharmaceutical composition injection of pantoprazole sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com