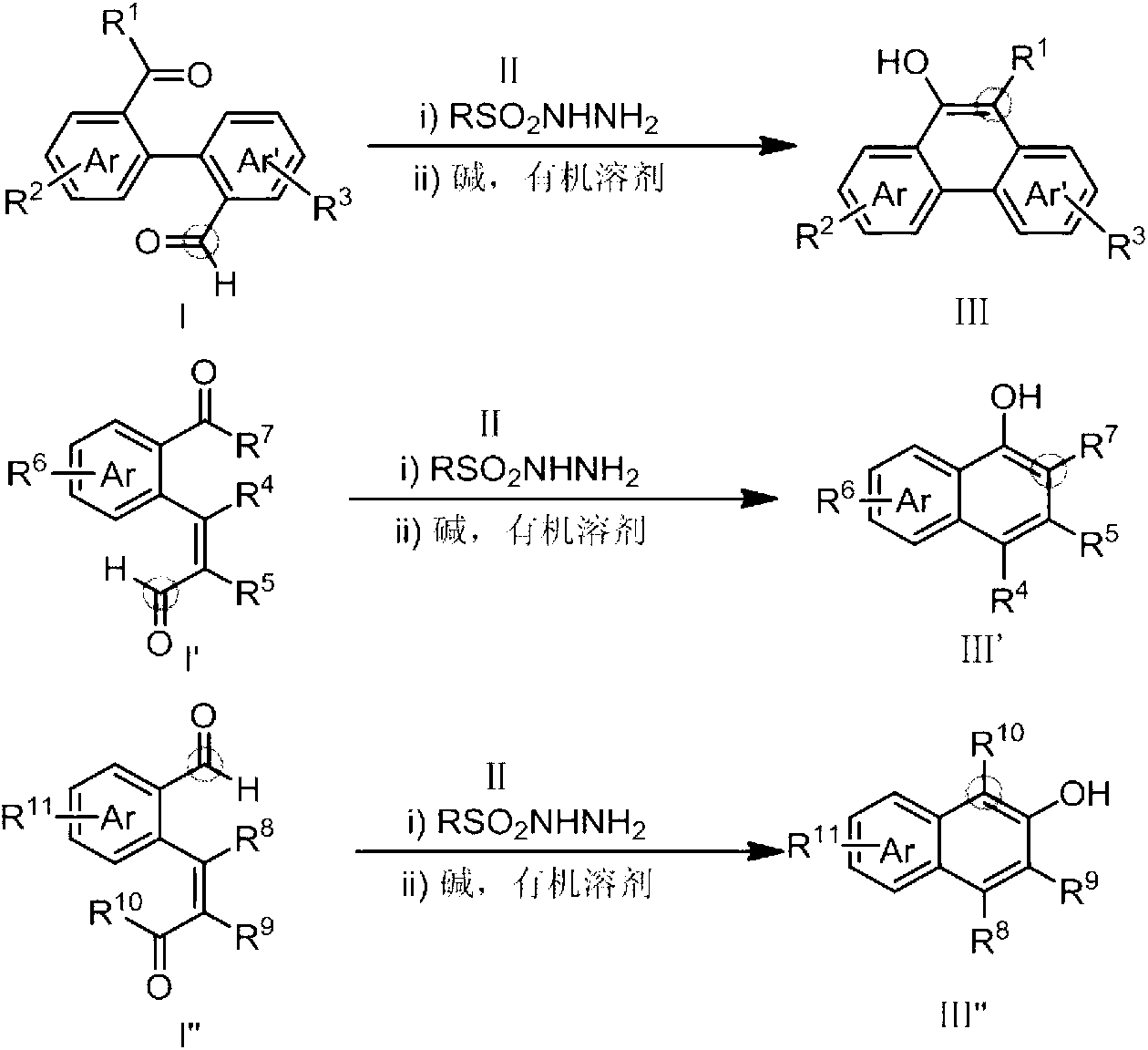
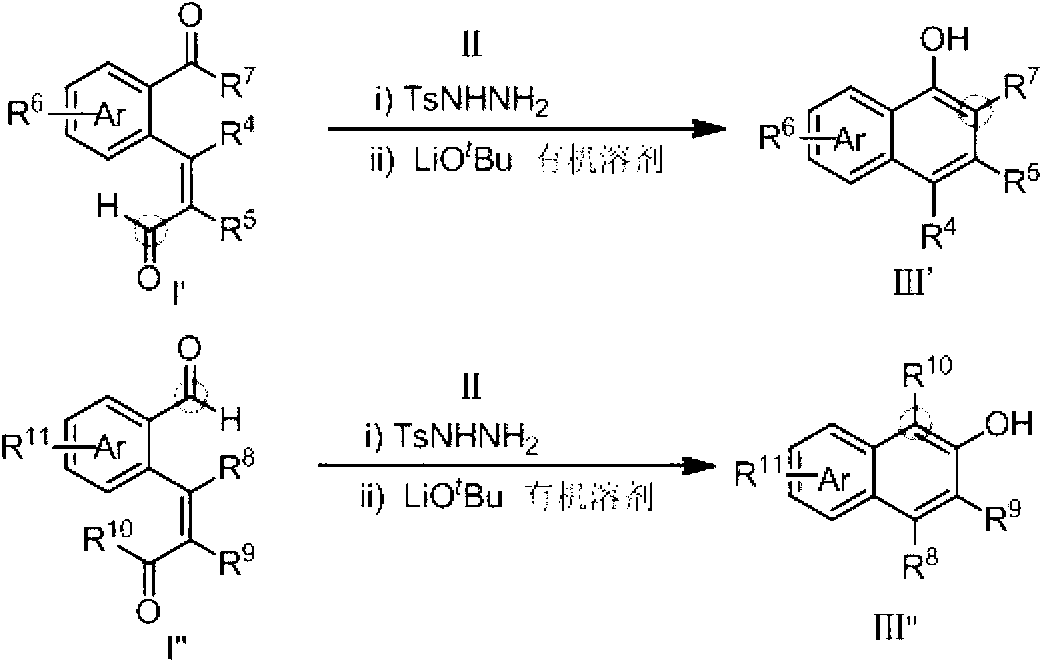
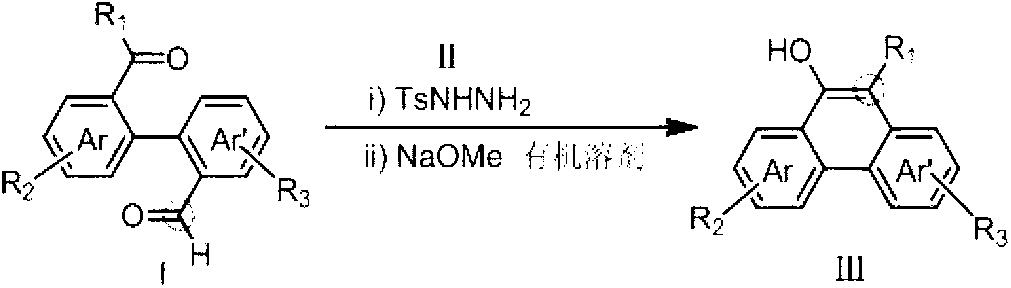Method for preparing hydroxy-substituted polycyclic aromatic compound
A technology of aromatic compounds and biscarbonyl compounds, applied in the field of organic synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0030] Example 1a Synthesis of 10-phenyl-9phenanthrene
[0031] Under the protection of argon, add 85.8mg (0.30mmol) of 1,1-biphenyl-2'-benzoyl-2-carboxaldehyde, 58mg (0.309mmol) of p-toluenesulfonate to the dried 25ml Schlenk bottle. Hydrazine, 6ml toluene. After reacting at 70°C for 30 min, NaOMe (41 mg, 0.75 mmol) was added, and the reaction was continued at 70°C for 30 min. AcOH (45mg, 0.75mmol, 2.5eq) was added to the system to quench the reaction and return to room temperature. After the reaction system is concentrated, the product can be obtained by purification with PE / EA=30:1 column chromatography. The reaction formula is as follows:
[0032]
[0033] The obtained compound is a pink solid with a yield of 96%. Its nuclear magnetic data is as follows:
[0034] 1 H NMR(400MHz, CDCl 3 )δ8.67(d, J=7.9Hz, 1H), 8.63(d, J=8.1Hz, 1H), 8.38(d, J=7.9Hz, 1H), 7.69-7.36(m, 10H), 5.45( s, 1H); 13 C NMR(100MHz, CDCl 3 )δ146.03,134.46,132.43,131.42,131.00,129.74,128.56,127.19,126.78,12...
Embodiment 1b
[0035] Example 1b Synthesis of 10-phenyl-9phenanthrene
[0036] Under the protection of argon, add 85.8mg (0.30mmol) of 1,1-biphenyl-2'-benzoyl-2-carboxaldehyde, 58mg (0.309mmol) of p-toluenesulfonate to the dried 25ml Schlenk bottle. Hydrazine, 10ml toluene. After reacting at 70°C for 30 min, NaOMe (41 mg, 0.75 mmol) was added, and the reaction was continued at 70°C for 30 min. AcOH (45mg, 0.75mmol, 2.5eq) was added to the system to quench the reaction and return to room temperature. After the reaction system is concentrated, the product can be obtained by purification with PE / EA=30:1 column chromatography. The reaction formula is as follows:
[0037]
[0038] The obtained compound is a pink solid with a yield of 96%. Its nuclear magnetic data is as follows:
[0039] 1 H NMR(400MHz, CDCl 3 )δ8.67(d, J=7.9Hz, 1H), 8.63(d, J=8.1Hz, 1H), 8.38(d, J=7.9Hz, 1H), 7.69-7.36(m, 10H), 5.45( s, 1H); 13 C NMR(100MHz, CDCl 3 )δ146.03,134.46,132.43,131.42,131.00,129.74,128.56,127.19,126.78,1...
Embodiment 1c
[0040] Example 1c Synthesis of 10-phenyl-9phenanthrene
[0041] Under the protection of argon, add 85.8mg (0.30mmol) of 1,1-biphenyl-2'-benzoyl-2-carboxaldehyde, 58mg (0.309mmol) of p-toluenesulfonate to the dried 25ml Schlenk bottle. Hydrazide, 15ml toluene. After reacting at 70°C for 30 min, NaOMe (41 mg, 0.75 mmol) was added, and the reaction was continued at 70°C for 30 min. AcOH (45mg, 0.75mmol, 2.5eq) was added to the system to quench the reaction and return to room temperature. After the reaction system is concentrated, the product can be obtained by purification with PE / EA=30:1 column chromatography. The reaction formula is as follows:
[0042]
[0043] The obtained compound is a pink solid with a yield of 96%. Its nuclear magnetic data is as follows:
[0044] 1 H NMR(400MHz, CDCl 3 )δ8.67(d, J=7.9Hz, 1H), 8.63(d, J=8.1Hz, 1H), 8.38(d, J=7.9Hz, 1H), 7.69-7.36(m, 10H), 5.45( s, 1H); 13 C NMR(100MHz, CDCl 3 )δ146.03,134.46,132.43,131.42,131.00,129.74,128.56,127.19,126.78,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com