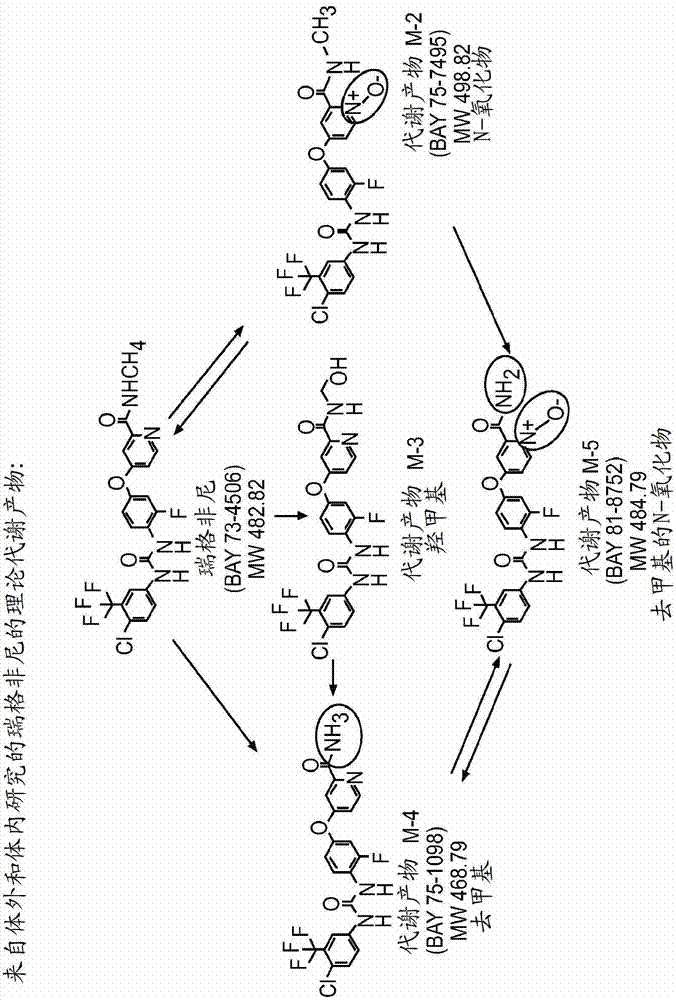
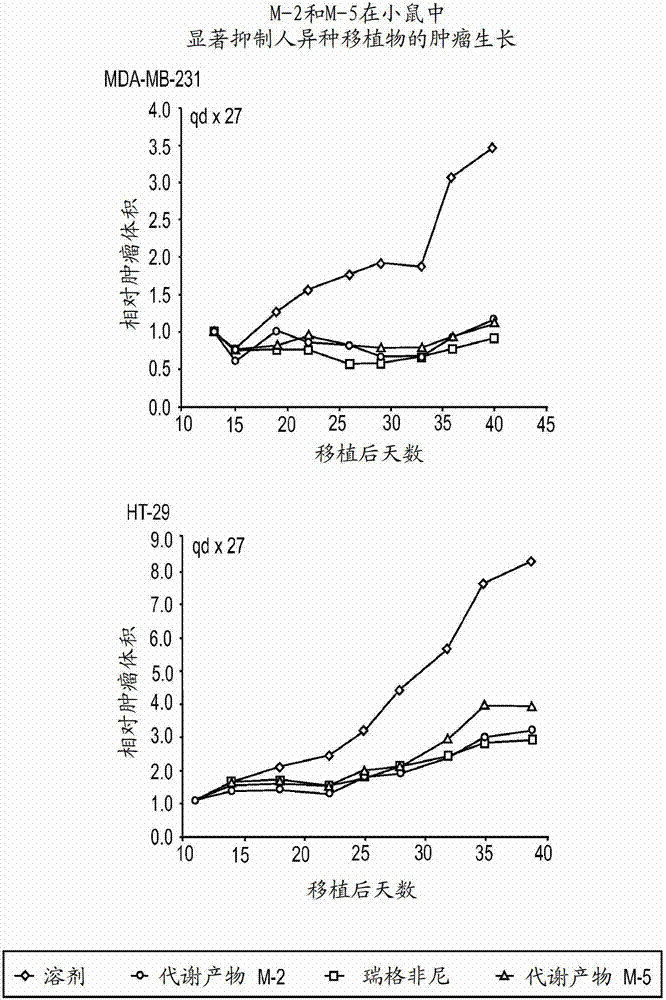
Synthetic metabolites of fluoro substituted omega-carboxyaryl diphenyl urea for the treatment and prevention diseases and conditions
A technology for anabolism and metabolites, which can be used in the fields of active ingredients of heterocyclic compounds, resistance to vector-borne diseases, drug combinations, etc., and can solve problems such as non-maintenance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0427] Example 1: Preparation of 4{4-[3-(4-chloro-3-trifluoromethylphenyl)-ureido]-3-fluorophenoxy}-pyridine-2-carboxylic acid carboxamide
[0428]
[0429]To a solution of 4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid carboxamide (177 mg, 0.68 mmol) in toluene (3 mL) was added 4-chloro-3-(trifluoromethyl ) phenylisocyanate (150mg, 0.68mmol). The mixture was stirred at room temperature for 72 hours. The reaction was concentrated under reduced pressure and the resulting residue was triturated with ether. The resulting solid was collected by filtration and dried under vacuum for 4 hours to obtain the title compound (155 mg, 0.32 mmol; 47% yield); 1H-NMR (DMSO-d6) 2.78 (d, J=4.9, 3H) , 7.03-7.08(m,1H), 7.16(dd,J=2.6,5.6,1H), 7.32(dd,J=2.7,11.6,1H), 7.39(d,J=2.5,1H), 7.60(s ,2H), 8.07–8.18(m,2H), 8.50(d,J=5.7,1H), 8.72(s,1H), 8.74-8.80(m,1H), 9.50(s,1H); MS (HPLC / ES)483.06m / z=(M+1).
Embodiment 2
[0430] Example 2: 4{4-[3-(4-chloro-3-trifluoromethylphenyl)-ureido]-3-fluorophenoxy}-pyridine-2-carboxylic acid formamide hydrochloride preparation
[0431] The free base form of the compound described in Example 1 (2.0 g) was dissolved in anhydrous THF (15 mL) and 4M HCl / dioxane (excess) was added. The solution was then concentrated under vacuum to yield 2.32 grams of an off-white solid. The crude salt was dissolved in hot ethanol (125 mL), activated carbon was added and the mixture was heated at reflux for 15 minutes. The hot suspension was filtered through a pad of Celite 521 and cooled to room temperature. The flask was placed in the refrigerator overnight. The crystalline solid was collected by suction filtration, washed with ethanol, then hexane and air dried. The mother liquor was concentrated and crystallization (in refrigerator) was performed overnight. A second crop of solids was collected and combined with the first crop. The colorless salt was dried in a vacu...
Embodiment 3
[0435] Example 3: 4{4-[3-(4-Chloro-3-trifluoromethylphenyl)-ureido]-3-fluorophenoxy}-pyridine-2-carboxylic acid carboxamide methanesulfonate preparation of
[0436] The free base form of the compound described in Example 1 (2.25 g) was dissolved in ethanol (100 mL) and the methanesulfonic acid stock solution (excess) was added. The solution was then concentrated in vacuo to give a yellow oil. Ethanol was added and concentrated again to afford 2.41 g of an off-white solid. The crude salt was dissolved in hot ethanol (about 125 mL) and then cooled slowly to crystallize. After reaching room temperature, the flask was placed in the refrigerator overnight. The colorless crystalline material was collected by suction filtration; the resulting filter cake was washed with ethanol, then hexane and air dried to give 2.05 g of material which was dried in a vacuum oven at 60°C overnight.
[0437] Melting point: 231°C
[0438] Elemental analysis:
[0439]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com