Application of flavan compound in preparing anti-complement medicines
A kind of compound and flavan technology, which is applied in the new application field of flavan compounds in the preparation of anti-complement drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 prepares flavan compound
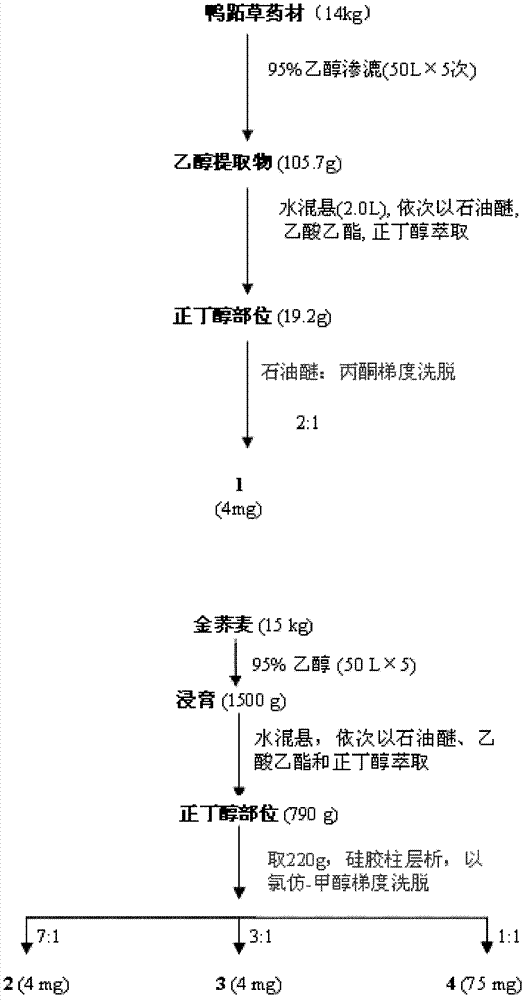
[0029] (1) Take 14kg of Commelina chinensis, cold-soak with ethanol at room temperature (50L×5 times), combine the extracts and concentrate until there is no alcohol smell, add water to dilute the extracts to 2L, and use petroleum ether, ethyl acetate, n-butyl Alcohol extraction (each 2 L x 3 times), the combined n-butanol extracts were concentrated to dryness to obtain 19.2 g of n-butanol extracts. The n-butanol part was subjected to silica gel column chromatography, eluted with petroleum ether (60-90°C), petroleum ether-acetone, and acetone gradient, and the fraction was purified by repeated column chromatography and SephadexLH-20 to obtain flavan compounds (1). Specific steps are as follows:
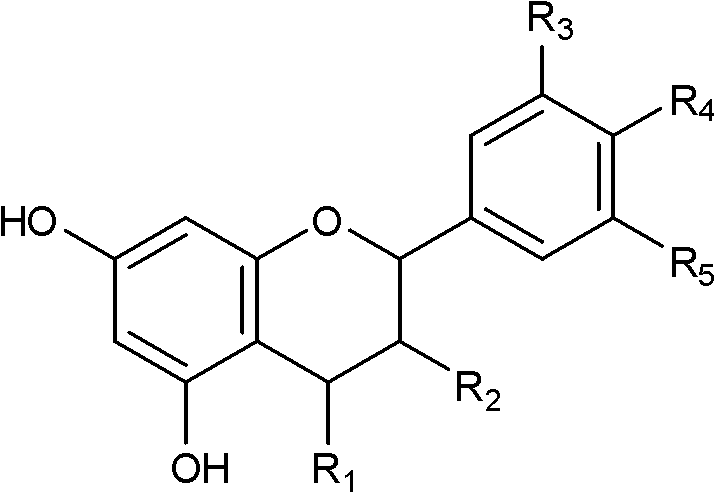
[0030] The obtained fraction was eluted with petroleum ether-acetone (2:1), and purified by Sephadex LH-20 to obtain compound 1 (4 mg). Using spectral analysis, its structure was determined to be p-catechin-3-O-β-D-(2-cinnamoyl)-glucopy...
Embodiment 2
[0035] Example 2 Anti-complement classical pathway test in vitro
[0036] Take 0.1ml of complement (guinea pig serum), add BBS to prepare a 1:5 solution, and dilute it to 1:10, 1:20, 1:40, 1:80, 1:160, 1:320 and 1:320 with BBS 640 solution. Dissolve 1:1000 hemolysin, 0.1ml of each concentration of complement and 2% SRBC in 0.3ml BBS, mix well, put in a low-temperature high-speed centrifuge after 30min in a 37°C water bath, and centrifuge at 5000rpm and 4°C for 10min. Take 0.2ml of the supernatant from each tube and place it in a 96-well plate, and measure the absorbance at 405nm. A full hemolysis group (0.1ml 2% SRBC dissolved in 0.5ml triple distilled water) was also set up in the experiment. The absorbance of three-distilled water lysed blood vessels was used as the standard of total hemolysis, and the hemolysis rate was calculated. Taking the dilution of complement as the X-axis, the percentage of hemolysis caused by each dilution of complement is plotted as the Y-axis. ...
Embodiment 3
[0037] Example 3 Anti-complement alternative pathway test in vitro
[0038] Take 0.2ml of complement (human serum), add AP diluent to prepare a 1:5 dilution solution, and double-dilute to 1:10, 1:20, 1:40, 1:80, 1:160, 1:320 and 1:640 solution. Take 0.15ml of complement of each concentration, 0.15ml of AP diluent and 0.20ml of 0.5% RE, mix well, place in a low-temperature high-speed centrifuge after 30 minutes in a 37°C water bath, and centrifuge at 5000rpm and 4°C for 10 minutes. Take 0.2ml of the supernatant from each tube and place it in a 96-well plate, and measure the absorbance at 405nm. At the same time, a complete hemolysis group (0.20ml 0.5% RE dissolved in 0.3ml triple distilled water) was set up in the experiment. The absorbance of three-distilled water lysed blood vessels was used as the standard of total hemolysis, and the hemolysis rate was calculated. Taking the dilution of complement as the X-axis, the percentage of hemolysis caused by each dilution of compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com