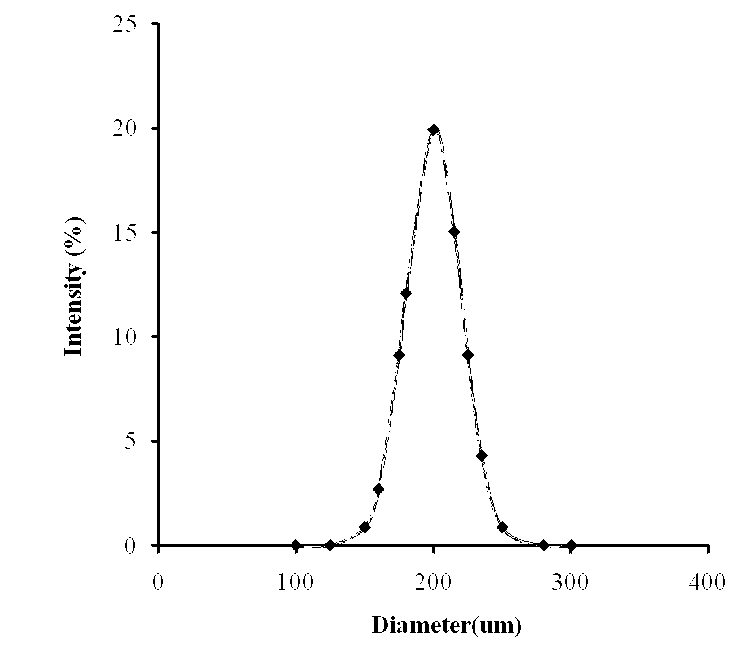
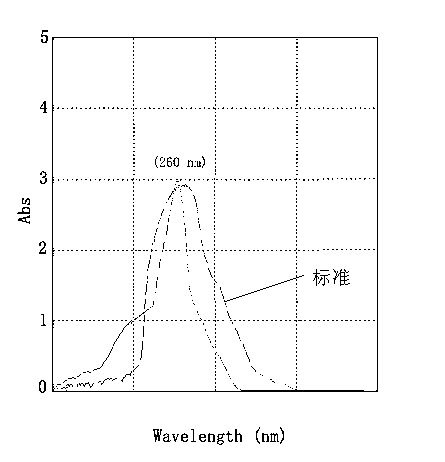
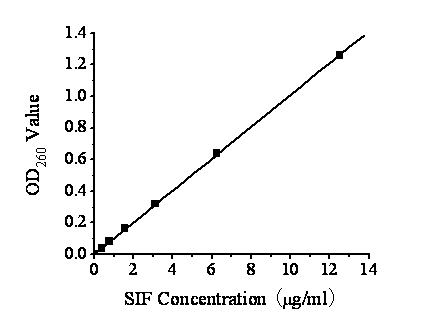
Preparation method of soybean isoflavone-chitosan slow-release microcapsules
A technology of soybean isoflavones and chitosan, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of low absorption rate, complicated operation, limited effect, etc. The effect of stable quality, uniform particles and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The preparation method of soybean isoflavone-chitosan sustained-release microcapsules comprises the following steps:
[0024] 1) prepare A liquid, A liquid is the calcium chloride chitosan gel liquid containing acetic acid, calcium chloride, chitosan, calcium chloride, chitosan, acetic acid are fully dissolved in distilled water successively during preparation;
[0025] 2) To prepare liquid B, mix the mixed sol solution containing sodium alginate with a mass concentration of 1%-3% and the mass-volume concentration of 10mg / ml-40mg / ml soybean isoflavones, and stir the mixed sol solution evenly with a stirrer Get liquid B;
[0026] 3) Gelation reaction, drop liquid B into liquid A by means of centrifugal granulation, trigger gelation reaction of liquid B, and generate soybean isoflavone-chitosan sustained-release microcapsules.
Embodiment 1
[0028] 1. Preparation of liquid A: First, pour 2L of single distilled water into a large beaker, and use a magnetic stirrer to fully dissolve in the order of anhydrous calcium chloride (20g), chitosan (10g), and acetic acid (20mL) in 2L single distilled water (dissolve calcium chloride fully first, then add chitosan to fully dissolve).
[0029] 2. Prepare liquid B: first pour 2L of single distilled water into a large beaker, then weigh 30g of sodium alginate, and dissolve it in 2L of single distilled water. The dissolution process needs to be heated in a water bath, and the temperature is controlled within the range of 60°C to 70°C. During the heating process, it should be fully stirred and emulsified until there are no granular particles and bubbles in the solution; measure 500mL of sodium alginate solution and pour it into a beaker, add 15g of soybean isoflavones that have been weighed, and stir well with a glass rod , until there are no small drug particles in the solution...
Embodiment 2
[0036] The difference from Example 1 is that when preparing liquid B, the selected concentration of sodium alginate is 1.00%: the relative yield of the obtained soybean isoflavone-chitosan sustained-release microcapsules is 79.5%, and the encapsulation efficiency is 62.5% , the drug loading was 23.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com