Facile and effective method of preparing 1,4-Bis(chlorodifluoromethyl)benzene
一种氯二氟甲基、二氟甲基的技术,应用在批次式或连续式用以制备1领域,能够解决产率低、复杂纯化步骤、反应时间长等问题,达到反应时间短、降低制作成本、成本低的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
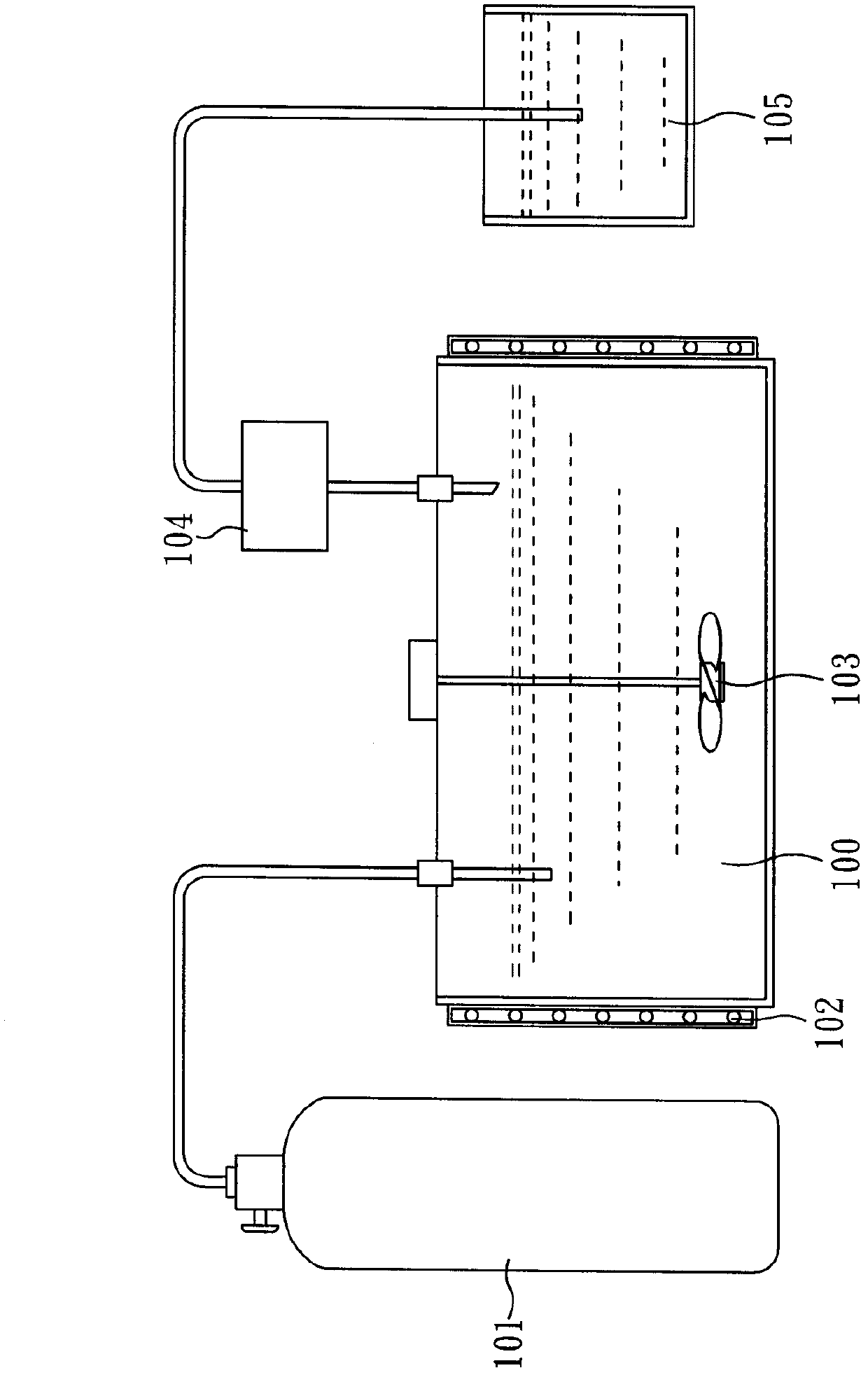
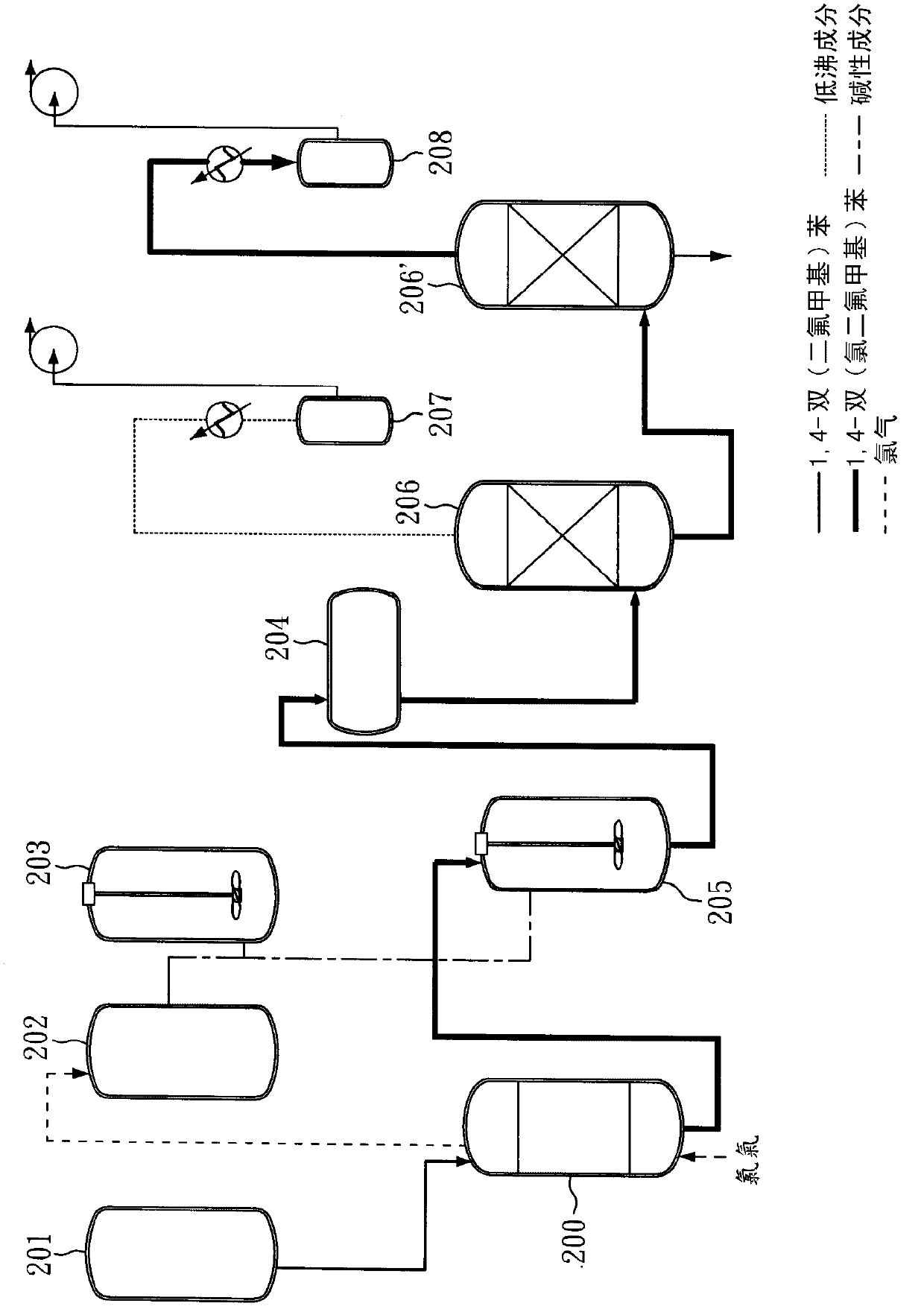
[0044] The method for preparing 1,4-bis(chlorodifluoromethyl)benzene provided by the invention comprises: (A) providing a method comprising 1,4-bis(difluoromethyl)benzene (1,4-Bis( difluoromethyl)benzene) reaction solution; (B) feed chlorine gas (Cl) at a temperature of 50-90°C 2 ) to the reaction solution, and (C) maintaining the reaction system pressure greater than 1 atm, to obtain 1,4-bis(chlorodifluoromethyl)benzene.
[0045] In the preparation method of the present invention, under a reaction pressure slightly greater than 1 atm, 1,4-bis(difluoromethyl)benzene can be directly reacted with chlorine, and there is no need to 1,4-bis(difluoromethyl)benzene Methyl)benzene is soluble in a solvent, and there is no need to add a photoinitiator, and 1,4-bis(difluoromethyl)benzene can react with chlorine gas under direct light. Since the preparation method of the present invention uses the reactant 1,4-bis(difluoromethyl)benzene as a solvent and reacts completely, there is no nee...
Embodiment 1
[0065] First, 300 g (1.69 mole) of 1,4-bis(difluoromethyl)benzene (TFPX) was added into a glass reactor, stirred evenly with a stirrer, and the reaction temperature was heated to 60° C. The glass reactor was then irradiated with a 400-watt mercury lamp.
[0066] Afterwards, a slight excess of Cl was continuously introduced 2 (approximately 336g, 4.73mole) into a glass reactor for chlorination. During the reaction process, the reaction pressure was maintained slightly higher than 1 atm (about 1.01 atm). After the reaction was continued for 45 minutes, a slightly yellow reactant was obtained. The purity of GC analysis was as high as 99.32%. The yellow reactant was neutralized with lye, and the oil and water phases were separated. And after steps such as distillation (pressure 10torr, b.p.125 ℃), obtain colorless liquid 408g, yield rate is 98.1%.
[0067] The liquid was analyzed by GC, and it was confirmed that the product was 1,4-bis(chlorodifluoromethyl)benzene with a purity ...
Embodiment 2
[0069] First, take 600.01g (3.37mole) of 1,4-bis(difluoromethyl)benzene (TFPX) into the glass reactor, stir evenly with a stirrer, and heat the reaction temperature to 60°C by the water-proof heating method , and then irradiate the glass reactor with a 400-watt mercury lamp.
[0070] Afterwards, a slight excess of Cl was continuously introduced 2 (about 600g, 8.43mole) into a glass reactor for chlorination. During the reaction process, the reaction pressure was maintained slightly higher than 1 atm (about 1.01 atm). After the reaction was continued for 100 minutes, a yellowish liquid was formed.
[0071] After completing the above steps, the light yellow liquid was subjected to GC analysis, and it was confirmed that the product of 1,4-bis(chlorodifluoromethyl)benzene was produced, and the purity was as high as 99.49%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com