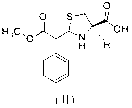
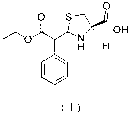
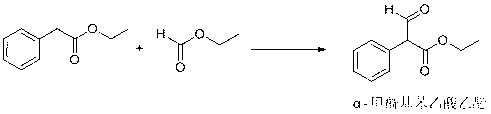Preparation method of leucongen
A technology of Likejun and ethyl phenylacetate, which is applied in the technical field of synthesizing high-purity medicinal Likejun, to achieve the effect of simplifying the process and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of ethyl α-formylphenylacetate.
[0026] In a 50 L reactor, add 35 L of isopropyl ether and sodium tert-butoxide (586 g, 6.1 mol), start stirring, and mix well. Cool in a water bath, control the internal temperature below 10°C, weigh and mix evenly. A mixture of 1500 g (9.15 mol) of ethyl phenylacetate and 725 g (9.80 mol) of ethyl formate was added dropwise to control the internal temperature below 10°C. After the dropwise addition, raise the temperature, control the internal temperature to 15°C, and keep it warm for 5 hours. The reaction liquid is finally a milky white suspension. Add 20L of purified water to dissolve, extract twice with 20L of isopropyl ether, take the water phase, and adjust the pH with dilute hydrochloric acid. The value was 1.0, the temperature was lowered to 0-5° C. to crystallize, and 1350 g was obtained with a yield of 76.8%. Melting point: 58.6-60.7°C. 1 H-NMR (300MHz, DMSO-d6): δ: 10.95(1H,s,CHO), 7.86(1H,s,Ar-CH), 7....
Embodiment 2
[0027] Example 2: Synthesis of ethyl α-formylphenylacetate.
[0028] In a 50 L reactor, add 35 L of methyl tert-butyl ether and potassium tert-butoxide (784 g, 7.0 mol), start stirring, and mix well. Cool in a water bath, control the internal temperature below 10°C, weigh and mix evenly. A mixture of 1500 g (9.15 mol) of ethyl phenylacetate and 725 g (9.80 mol) of ethyl formate was added dropwise to control the internal temperature below 10°C. After the dropwise addition, raise the temperature, control the inner temperature to 20°C, and keep it warm for 4.5 hours. The reaction liquid is finally a milky white suspension. Add 20L of purified water to dissolve it, extract it twice with 20L of methyl tert-butyl ether, take the water phase, and use dilute Adjust the pH value to 2.0 with hydrochloric acid, cool down to 0-5 degrees to crystallize, and obtain 1280g with a yield of 72.9%. Melting point: 59.1-61.2 °C.
Embodiment 3
[0029] Example 3: Synthesis of ethyl α-formylphenylacetate.
[0030] In a 50 L reactor, add 15 L of isopropyl ether, 20 L of methyl tert-butyl ether and sodium tert-butoxide (586 g, 6.1 mol), start stirring, and mix well. Cool in a water bath, control the internal temperature below 10°C, weigh and mix evenly. A mixture of 1500 g (9.15 mol) of ethyl phenylacetate and 725 g (9.80 mol) of ethyl formate was added dropwise to control the internal temperature below 10°C. After the dropwise addition, raise the temperature, control the internal temperature to 15°C, and keep it warm for 5 hours. The reaction liquid is finally a milky white suspension. Add 20L of purified water to dissolve, extract twice with 20L of isopropyl ether, take the water phase, and adjust the pH with dilute hydrochloric acid. The value was 1.0, the temperature was lowered to 0-5° C. to crystallize, and 1310 g was obtained with a yield of 74.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com