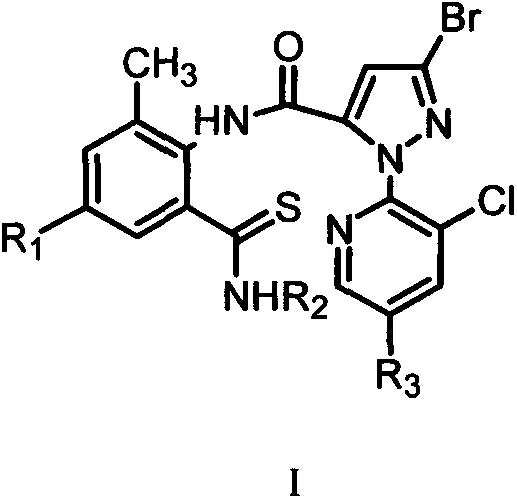
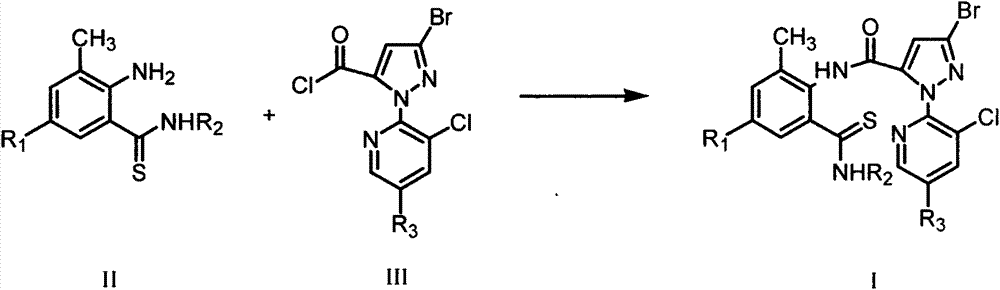
Thiobenzamide compounds and application thereof
An o-aminothiobenzamide and compound technology, which is applied in the field of thiobenzamide compounds, can solve the problem that the insecticidal activity of the thioamide compounds has not been disclosed, and achieves the improvement of effective utilization, reduction of influence, good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 compound 1
[0029] (1) Synthesis of 8-methyl-1H-[1,3]-benzoxazine-2,4-dione
[0030]
[0031] Into a 500mL reaction flask, add 15.1g (0.1mol) 2-amino-3-methylbenzoic acid, 150mL ethyl acetate, 0.5g pyridine, dropwise add 19.8g (0.067mol) Sanko Gas and 100mL of ethyl acetate, dropwise, stirred and reacted at 30-35°C for 4 hours, then raised the temperature and refluxed for 2 hours, removed all phosgene, cooled to room temperature, filtered with suction, and washed with water to obtain 15.9g of white solid, collected The rate is 89.8%.
[0032] (2) Synthesis of 2-amino-N,3-dimethylbenzamide
[0033]
[0034] Add 17.8g (0.1mol) of 8-methyl-1H-[1,3]-benzoxazine-2,4-dione, 100mL ethyl acetate, 1.5g glacial acetic acid in 15- Gradually add 15.5g (0.2mol) of 40% methylamine aqueous solution at 20°C, drop it, stir at room temperature for 2 hours, separate the liquids, leave to stand to separate the water layer, dry with anhydrous magnes...
Embodiment 2
[0045] The preparation of embodiment 2 compound 3
[0046] (1) Synthesis of 2-amino-3-methyl-5-chloro-N-allylthiobenzamide
[0047]
[0048] To a 500mL four-necked bottle, add P 2 S 5 22.2g (0.1mol), Na 2 CO 310.6g (0.1mol), ethyl acetate 200mL, stir at room temperature for 1h until the system is clear, add 22.45g (0.1mol) 2-amino-3-methyl-5-chloro-N-allylbenzamide in batches (prepared according to the method of Example 1), the temperature was raised to reflux for 6 hours, and after the reaction of the raw materials was complete, the temperature was lowered to room temperature, and 30 mL of water was added until the system was clarified, and the liquid was separated. The ethyl acetate layer was washed with a saturated saline solution (30 mL×2), and no Water Na 2 SO 4 After drying, the solvent was evaporated to dryness to obtain 17.3 g of an orange-yellow solid with a yield of 71.93%.
[0049] (2) Synthesis of compound 3
[0050]
[0051] Add 2.4g (0.01mol) 2-amin...
Embodiment 3
[0052] The preparation of embodiment 3 compound 7
[0053] (1) Synthesis of 2-amino-3-methyl-5-chloro-N-isopropylthiobenzamide
[0054]
[0055] To a 500mL four-necked bottle, add P 2 S 5 22.2g (0.1mol), Na 2 CO 3 10.6g (0.1mol), ethyl acetate 200mL, stirred at room temperature for 1h until the system was clear, added 2-amino-3-methyl-5-chloro-N-isopropylbenzamide (prepared according to the method in Example 1) in batches ) 22.65g (0.1mol), heat up to reflux for 6h, after the reaction of the raw materials is complete, cool down to room temperature, add 30mL of water until the system is clear, separate the layers, wash the ethyl acetate layer with saturated saline solution (30mL×2), anhydrous Na 2 SO 4 After drying, the solvent was evaporated to dryness to obtain 17.8 g of a yellow solid with a yield of 73.4%.
[0056] (2) Synthesis of compound 7
[0057]
[0058] Add 2.42g (0.01mol) 2-amino-3-methyl-5-chloro-N-isopropylthiobenzamide in 100mL reaction flask, 20mL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com