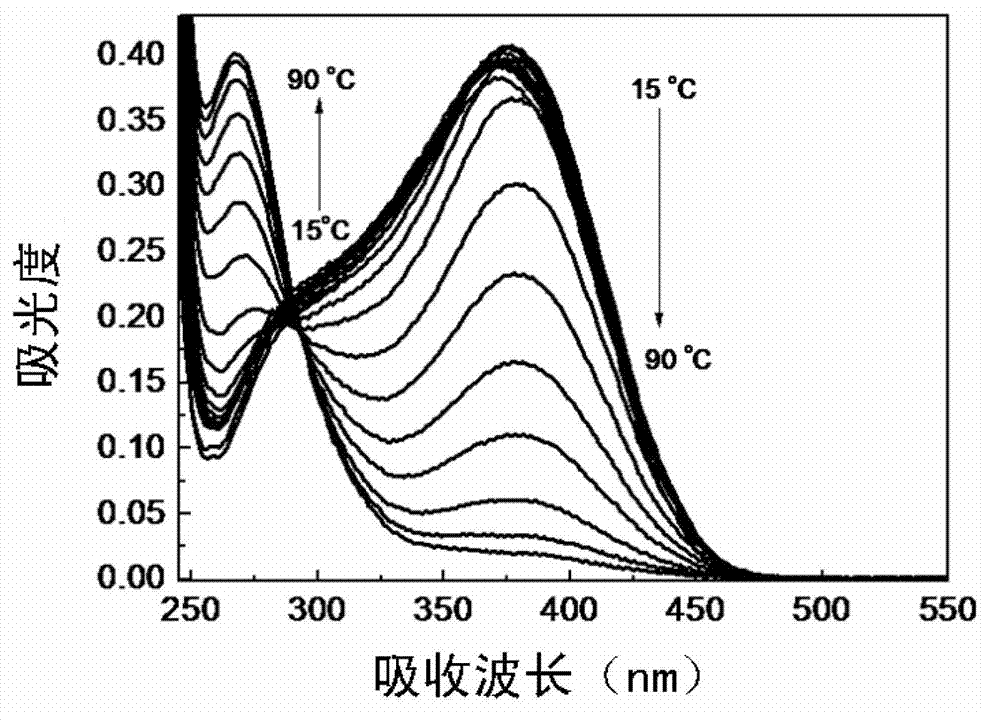
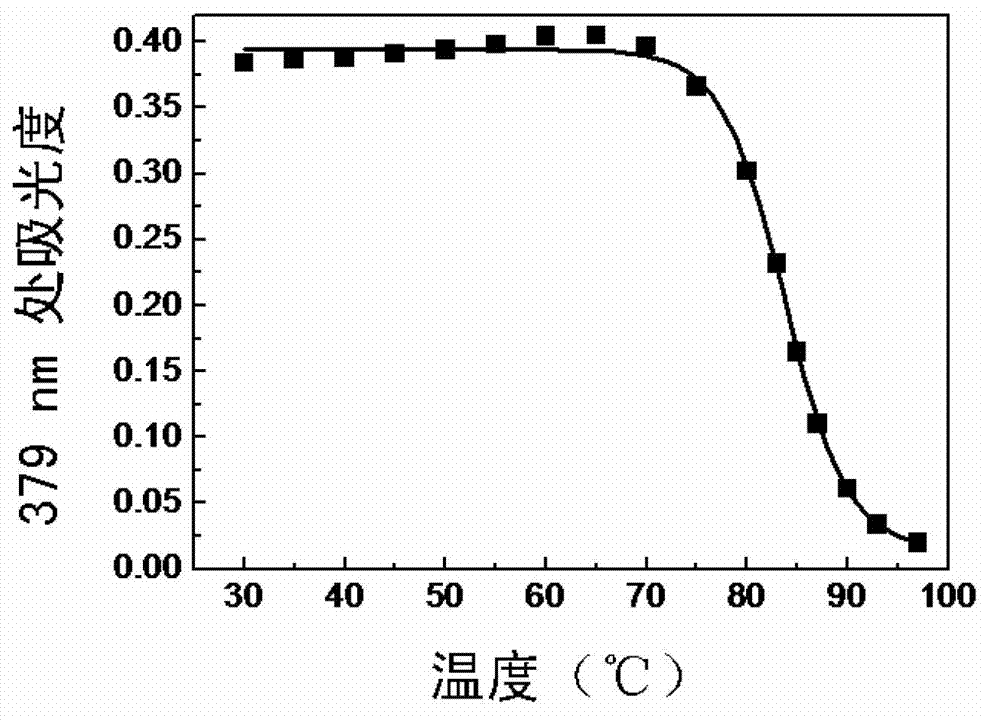
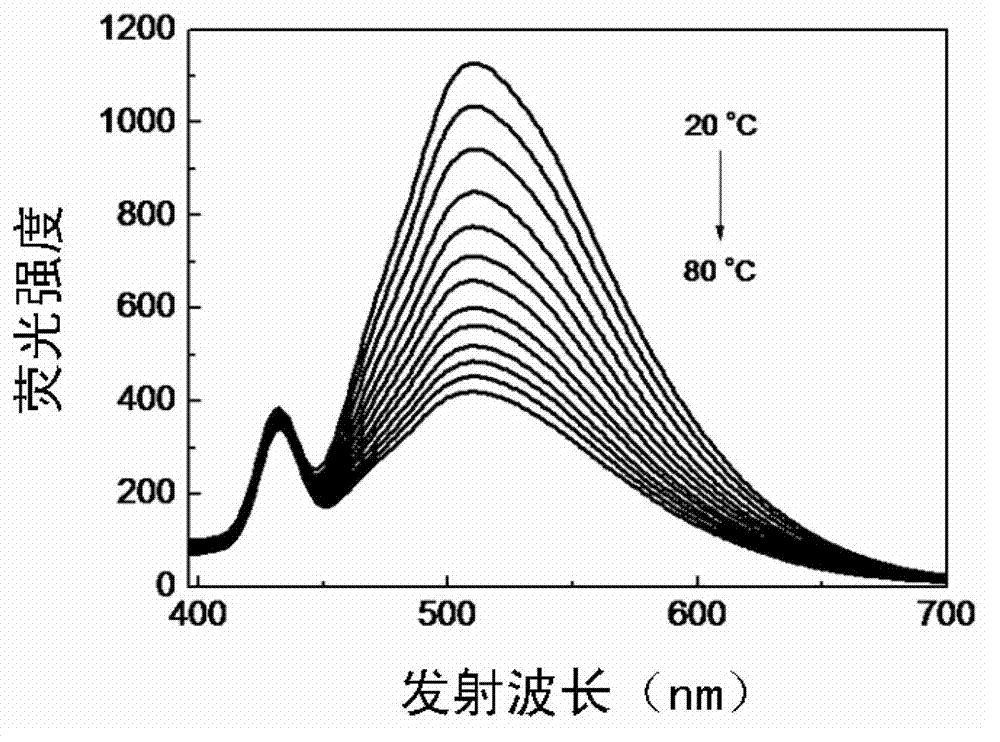
Preparation method for electrondrawing group containing semi-cyanine temperature-sensitive molecular optical probe
A technology of electron-withdrawing groups and optical probes, which is applied in the field of preparation of temperature-sensitive molecular optical probes containing electron-withdrawing groups hemicyanine, can solve problems such as limited applications, and achieve rapid measurement and monitoring effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Synthesis of 4-nitrophenylvinylindoline tetrafluoroborate (3a):
[0024]
[0025] Weigh 0.38g (0.0025mol) of p-nitrobenzaldehyde and place it in a 50mL round bottom flask, add 0.75g (0.0025mol) of N-methyl-2,3,3-trimethyl-3H-indolium iodide ) and 15mL of absolute ethanol, oil bath, stirring, and react at 100°C for 14 hours; then add 0.35g potassium tetrafluoroborate to the flask, and react for 2 hours; spin the solution to dryness, dissolve the solid in 5mL of DMF, and filter to remove inorganic salts , the obtained solution was spin-dried, and the solid was recrystallized with 10 mL of ethanol to obtain 0.52 g of orange crystals, with a yield of 52%. mp.228-230°C; ESI: m / z[M-BF 4 - ] + :307.1441; 1 H NMR (DMSO-d 6 ,400MHz), δ (ppm) 1.86 (s, 6H), 4.27 (s, 3H), 7.73-7.71 (m, J=2.2Hz, 2H), 7.94-7.90 (d, J=16.4Hz, 2H), 8.03-8.01 (t, J=4.4Hz, 1H), 8.46-8.43 (d, J=8.8Hz, 2H), 8.54-8.50 (m, J=16.4Hz, 3H); 13 C NMR (DMSO-d 6 ,100MHz), δ(ppm) 182.73, 150.0...
Embodiment 2
[0032] Example 2: Synthesis of 2,4-dinitrophenylvinylindoline tetrafluoroborate (3b):
[0033]
[0034] Weigh 0.29g (0.0015mol) of 2,4-dinitrobenzaldehyde into a 50mL round bottom flask, add 0.45 g (0.0015mol) and 15mL of absolute ethanol, oil bath, stirring, react at 100°C for 10 hours; then add 0.30g potassium tetrafluoroborate to the flask, and react for 2 hours. The solution was spin-dried, the solid was dissolved in 5 mL of DMF, the inorganic salt was removed by filtration, the obtained solution was spin-dried, and the solid was recrystallized with 10 mL of ethanol to obtain 0.22 g of dark red crystals with a yield of 33%. mp.214-216°C; ESI: m / z[M-BF 4 - ] + :352.1292; 1 H NMR (DMSO-d 6 ,400MHz), δ (ppm) 1.85 (s, 6H), 4.29 (s, 3H), 7.75-7.73 (t, J=3.6Hz, 2H), 7.86-7.82 (d, J=16.4Hz, 1H), 7.97-7.95 (t, J=4.2Hz, 1H), 8.06-8.04 (t, J=4.4Hz, 1H), 8.53-8.51 (d, J=8.4Hz, 1H), 8.80-8.77 (m, J= 8.4Hz, 2Hz, 2H), 8.91-8.90 (d, J=2Hz, 1H); 13 C NMR (DMSO-d 6 ,100MHz), δ(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com