Pseudo-polymorphic form of desloratadine citrate disodium and preparation method thereof
A technology for loratadine and pseudopolymorphism, which is applied to the field of pseudopolymorphism of desloratadine citrate and its preparation, can solve the problems of impossible prediction of drug formation, different crystal forms and the like, and achieves good stability And the effect of solubility, high stability and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of 8-chloro-6,11-dihydro-11-(4-piperidinylene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine
[0031] Add 100g of loratadine to a 500mL three-neck flask, add 300mL of hydrochloric acid with a mass fraction of 50%, heat up to 90°C, stir, and react for 3-4 hours. After the reaction, take a sample TLC to track the reaction process. After the reaction of the raw materials is completely stopped, cool to room temperature. Add 300mL of ice water to the solution, and slowly add 20% sodium hydroxide aqueous solution under ice bath conditions to adjust the pH value to 10. At this time, a large amount of white precipitates are precipitated in the solution, and the precipitates are filtered under reduced pressure with 300mL of acetic acid Ethyl ester dissolves. The filtrate was extracted twice with 200 mL ethyl acetate, the organic phase was mixed with the above ethyl acetate solution in which the precipitate was dissolved, dried with anhydrous sodium sulfate,...
Embodiment 2
[0032] Example 2 Preparation of 8-chloro-6,11-dihydro-11-(4-piperidinylene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine
[0033] Add 100g of loratadine to a 500mL three-neck flask, add 300mL of sulfuric acid with a mass fraction of 50%, heat up to 90°C, stir, and react for 5-6 hours. After the reaction, take a sample TLC to track the reaction process. After the reaction of the raw materials is completely stopped, cool to room temperature. Add 300mL ice water to the solution, and slowly add potassium hydroxide aqueous solution with a mass fraction of 20% under ice bath conditions to adjust the pH value to 12. At this time, a large amount of white precipitates are precipitated in the solution, and the precipitates are filtered under reduced pressure with 300mL toluene. dissolve. The filtrate was extracted twice with 200 mL of toluene, the organic phase was mixed with the above-mentioned toluene solution in which the precipitate was dissolved, dried with anhydrous sodium sulfat...
Embodiment 3
[0034] Example 3 Preparation of polymorphic compounds of desloratadine citrate
[0035] Add 28g of citric acid and 41g of 8-chloro-6,11-dihydro-11-(4-piperidinylene)-5H-benzo-[5,6] prepared in Example 1 into a 1000mL three-necked flask Add 300mL of methanol to -cycloheptane-[1,2-b]-pyridine, and slowly drop in NaOH aqueous solution (11g, 200mL of pure water) at 25°C with stirring, adjust the pH to 6-7, and continue stirring for 30min , a white solid appeared, continued to stir for 3-4h, more solids were precipitated, filtered under reduced pressure, and the filter cake was washed with 300mL. The obtained solid was dried under vacuum at 50° C. for 4 h to obtain 70 g of white solid. The yield is 75%.
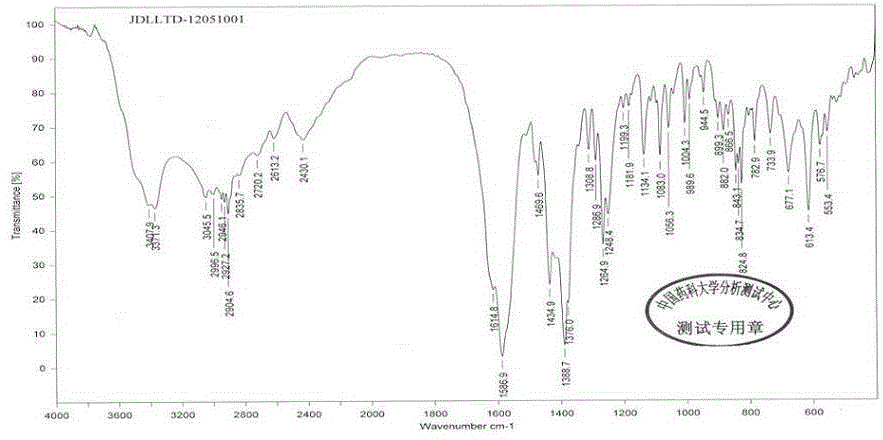
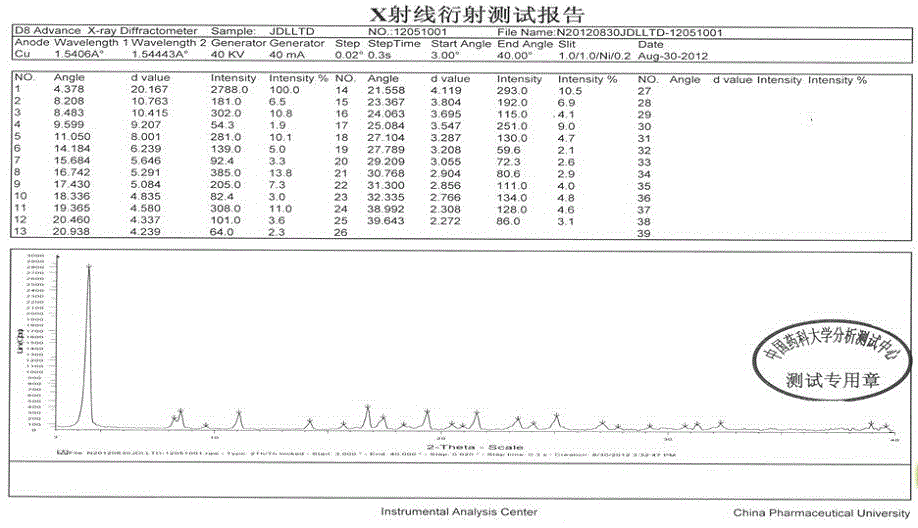
[0036] The crystal is used as a sample, and powder X-ray diffraction is measured to obtain figure 1 The X-ray powder diffraction pattern shown, wherein at about 4.378°, 8.208°, 8.483°, 11.050°, 14.184°, 16.742°, 17.430°, 19.365°, 21.558°, 23.367°, 25.084°, 27.104°, 32...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com