Solid supported gold nanoparticles, methods of use thereof, and methods for making same
A technology of gold nanoparticles and average particle size, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1. Catalytic activity screening
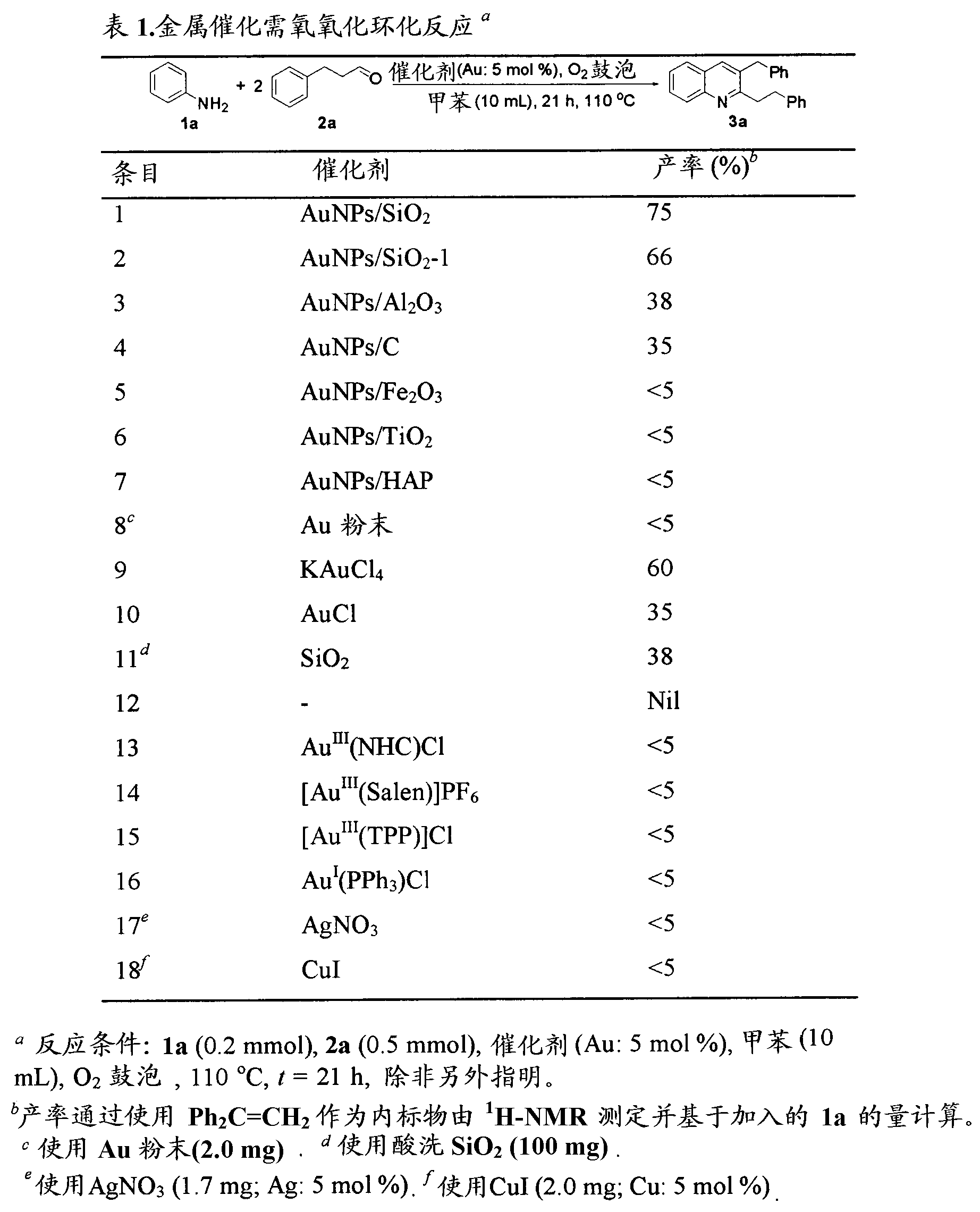
[0054] We give 3a for the oxidative cyclization of 1a and 2a using oxygen as the oxidant to screen the catalytic activity of various solid-supported AuNPs and gold salts (Table 1). Among various gold catalysts, AuNPs / SiO 2 is the most active catalyst (entry 1). For AuNPs / SiO prepared according to the Rossi method 2 Similar product yields were found for the -1 catalyst (entry 2). 3a The low product yields of 3a (entries 3–7) were obtained when other solid-supported AuNPs catalysts were used. It is worth noting that the reference catalyst AuNPs / Fe from the World Gold Council 2 o 3 (Sample No. 104C) and AuNPs / TiO 2 (Sample No. 168A) was not active in this oxidative cyclization reaction (entries 5-6). Loose gold powder (2-5 μm) was inactive under the reaction conditions used (entry 8). KAuCl 4 and AuCl gave moderate yields of 3a (entries 9-10), but they could not be recycled. It should be noted that SiO 2 3a alone gav...
Embodiment 2
[0056] Example 2. Recirculation experiment
[0057] AuNPs / SiO 2 It could be recovered by centrifugation and reused in seven consecutive runs without significant loss of reactivity (Table 2). After each successive operation, no significant changes in the average particle size and monodispersity of AuNPs were shown. After the seventh operation, AuNPs on SiO 2 The average particle size and monodispersity on the surface were 27.9±3.0nm and 10.8% ( image 3 ). The recovered AuNPs / SiO must be rinsed with piranha solution before each recycling 2 catalyst. Presumably, the acid treatment can remove the organic impurities capped on the surface of AuNPs and make the active Au δ+ Site regeneration. Indeed, X-ray photoelectron spectroscopy (XPS) analysis revealed that the binding energy increased from 83.9 eV to 84.4 eV after acid treatment (Au 4f 7 / 2 ), indicating that a larger fraction of Au δ+ matter on the surface of AuNPs.
Embodiment 3
[0058] Example 3. Synthesis of substituted quinolines
[0059] Next, we examine the "AuNPs / SiO 2 + O 2 The substrate scope of the scheme. As shown in Table 3, this scheme can efficiently catalyze the cyclization of various substituted anilines 1a-n with 2a, giving 3a-n in up to 95% product yield (entries 1-14). Good to excellent product yields were obtained when anilines with electron-donating substituents were used (entries 1-13). Cyclization of o-toluidine 1m containing two aniline groups was moderately The yield gave the corresponding biquinoline 3m (entry 13). With an electron donating substituent (CH 3 or OCH 3 ) gave better product yields than ortho- or para-substituted anilines (compare entries 4 and 10 with entries 3, 5 and 9). 1 n containing an electron-poor group gave low product yields (entry 14). Oxidative cyclization of 1d or 1j with 2a both gave the 7-isomer and 5-isomer in ratios of 6:1 (3d-7:3d-5) and 9:1 (3j-7:3j-5), respectively. isomers, and similar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com