Skin drug composition containing methylprednisolone aceponate and amino acid
A technology of methylprednisolone acetate and composition, which is applied in the field of pharmaceutical composition for skin, can solve the problems of great irritation and poor compliance, and achieve the effect of promoting absorption, good absorption and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
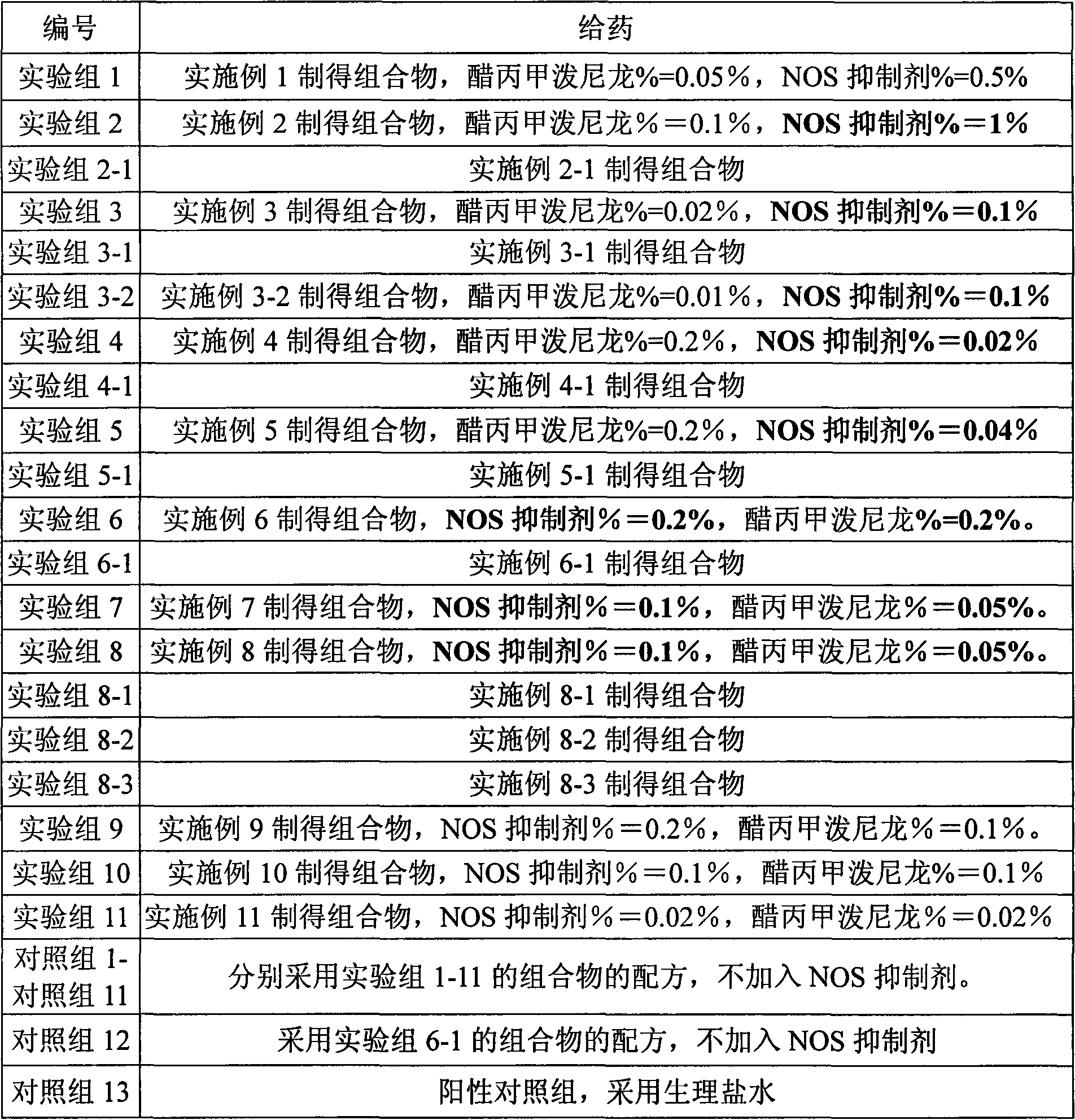
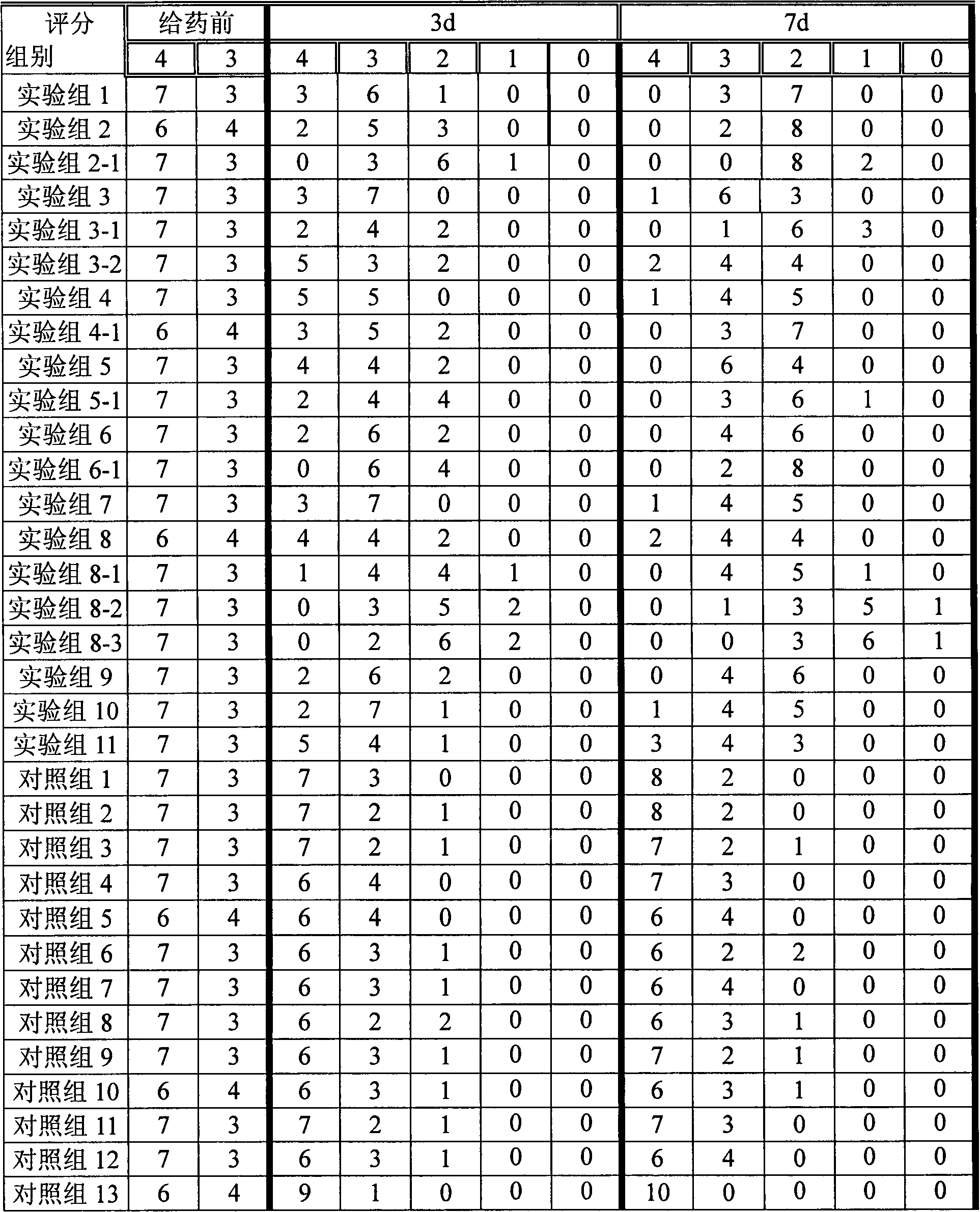
[0033] L-N6-(1-iminoethyl)-lysine 5g, appropriate amount of sodium chloride, sodium carboxymethylcellulose 2.5g, poloxamer 0.5g, methylprednisolone acetate 0.5g, the rest Water for Injection
[0034] Preparation method: Dissolve the carboxymethylcellulose sodium and poloxamer of the prescribed amount in 500ml water for injection, stir to dissolve, add the prescribed amount of L-N6-(1-iminoethyl)-lysine, Adjust the pH to 5.5. Add the prescribed amount of methylprednisolone acetone micropowder, and add the remaining amount of water for injection. Adjust to isotonicity with sodium chloride and dispense. The obtained composition NOS inhibitor %=0.5%, methylprednisolone acetate %=0.05%.
Embodiment 2
[0035] Embodiment 2 gel
[0036] N-monomethyl-L-arginine 10g, glycerol 50ml, carbomer 9342g, methylprednisolone acetate 1g,
[0037] Take the prescribed amount of Carbomer 934 and add glycerol to moisten and grind, add 500ml of purified water to swell as a gel matrix, add the prescribed amount of acepromethylprednisolone micropowder into the gel matrix and stir evenly, and then add the prescribed amount N-monomethyl-L-arginine, dissolved in an appropriate amount of water, slowly added to the gel matrix and stirred evenly, adjusted the pH value to 5.5 with an appropriate amount of 1mol / L sodium hydroxide, and added the rest of the water have to. The obtained composition has NOS inhibitor %=1%, methylprednisolone acetone=0.1%.
Embodiment 2-1
[0039] Prescription is identical with embodiment 2, gets menthol 10g in addition, dissolves in the prescription quantity glycerol, other preparation methods are identical with embodiment 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com