TAT-LBD-PEP fusion protein and application of TAT-LBD-PEP fusion protein in treatment of central nervous system lesion
A technology of fusion protein and central nervous system, which is applied in the field of protein transduction, can solve the problems such as difficult to reach the therapeutic concentration of drugs, and achieve the effect of enhancing targeting, reducing side effects and increasing drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
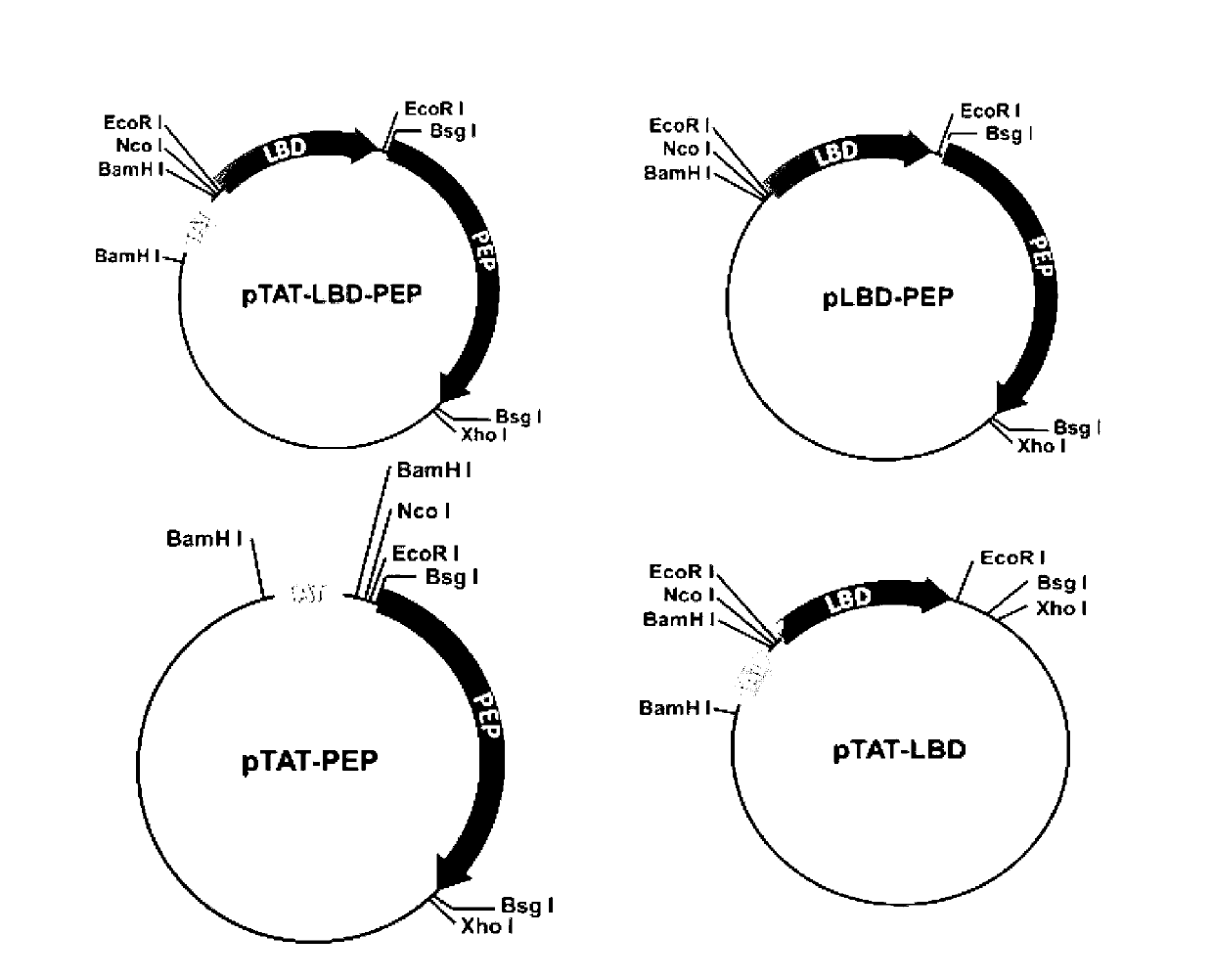
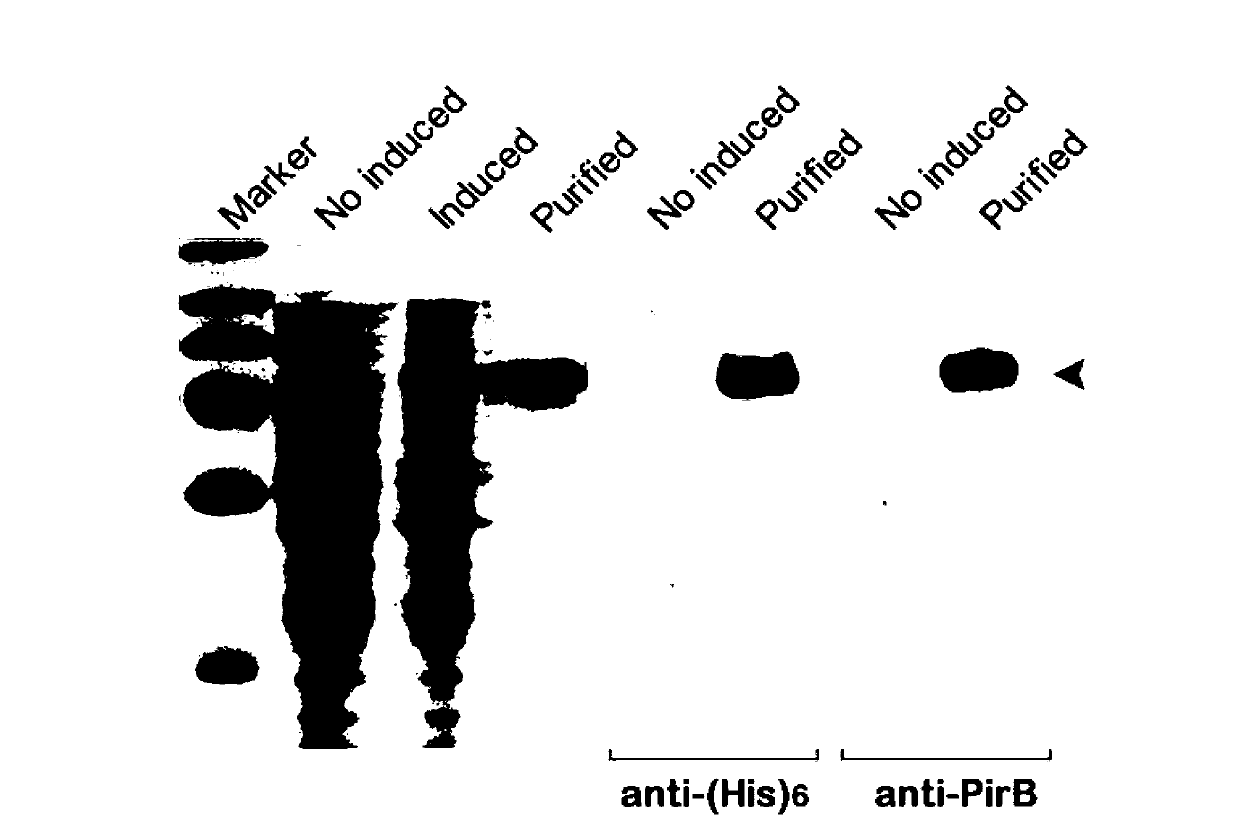
[0031] Example 1: Construction, expression and identification of TAT-LBD-PEP fusion protein
[0032] The TAT-LBD-PEP fusion protein is prepared by fusing TAT and LBD-PEP, which specifically includes the following steps:
[0033] 1. Obtain the LBD gene and PEP sequence from Genebank, then connect the LBD gene and the PEP sequence through the GGCGGTGGCGGTTCA sequence to synthesize the LBD-PEP gene sequence, and make the two ends of the LBD-PEP gene sequence contain Nco I and Xho I sequences respectively , the gene sequence of the entire LBD-PEP is:
[0034] LBD-PEP cDNA sequence:
[0035] A CCATGG CC–CTGCCGGGCGCATCGGGCACCTGTCCGGAACGCGCACTGGAACGTCGTGAAGAAGAAGCAAATGTTGTCCTGACGGGTACGGTTGAAGAAATTCTGAACGTTGATCCGGTCCAGCATACCTATAGCTGCAAAGTCCGTGTGTGGCGCTACCTGAAAGGCAAGGATCTGGTGGCACGTGAAAGTCTGCTGGACGGCGGTAATAAGGTGGTTATTTCCGGCTTTGGTGATCCGCTGATCTGTGACAACCAAGTCAGTACCGGTGATACGCGCATCTTTTTCGTTAATCCGGCACCGCCGTATCTGTGGCCGGCACATAAAAACGAACTGATGCTGAATAGCTCTCTGATGCGTATTACGCTGCGCAACCTGGAAGAAGTGGAAT...
Embodiment 2
[0049] Example 2: Drug efficacy test of TAT-LBD-PEP fusion protein
[0050] (1) Identification of toxicity and transduction function of TAT-LBD-PEP fusion protein
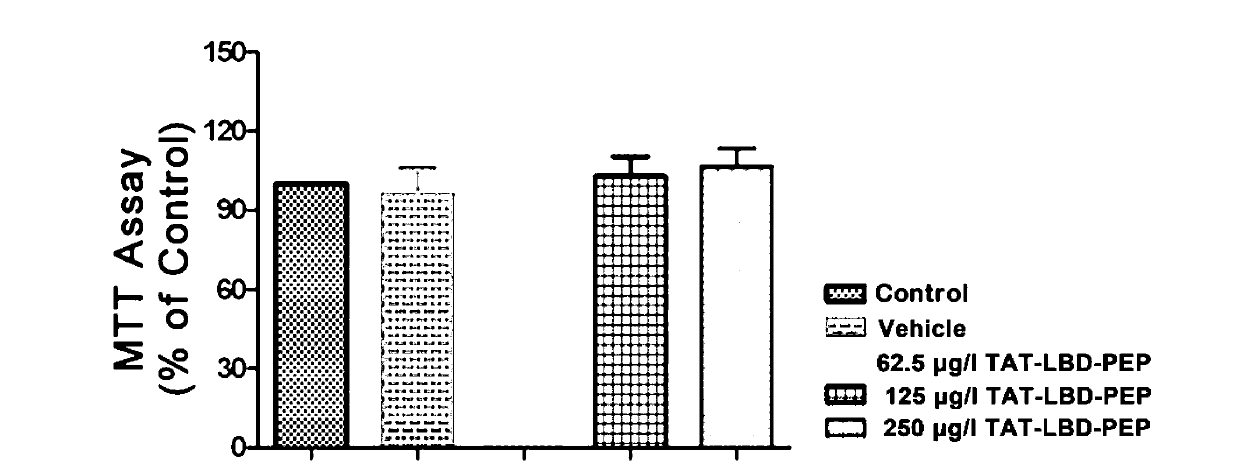
[0051] 1. Toxicity analysis of different concentrations of TAT-LBD-PEP on primary neurons
[0052] (1) Primary culture of hippocampal neurons until day 5, adding purified fusion protein TAT-LBD-PEP (62.5 μg / l, 125 μg / l, 250 μg / l) and solvent (PBS);
[0053] (2) For normal culture, perform cell viability analysis (MTT method) at 12h, 24h, and 48h after adding protein.
[0054] 2. Identification of TAT-LBD-PEP fusion protein transduction and targeting
[0055] (1) Mouse model of global cerebral ischemia: BCCAO preparation;
[0056] (2) Animal grouping: TAT-LBD-PEP, TAT-PEP injection group, PBS injection group;
[0057] (3) Inject the above protein by intraperitoneal injection of 1 mg / kg and PBS into mice;
[0058] (4) At different times (6h, 12h, 24h) after injection, they were executed under anesthesia, fixed, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com