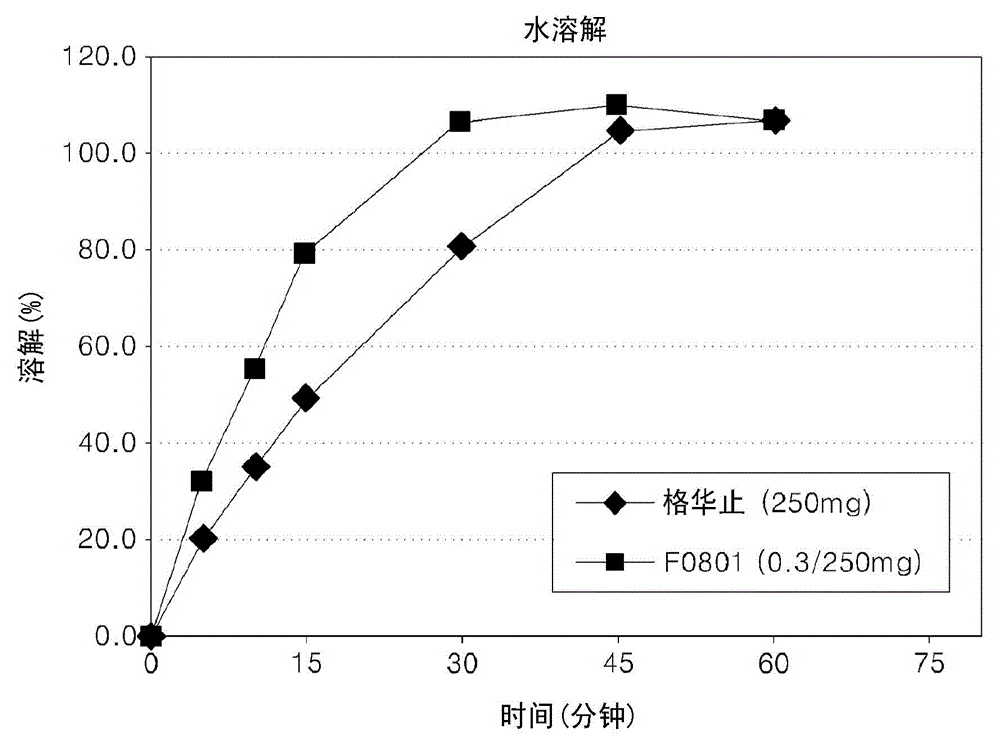
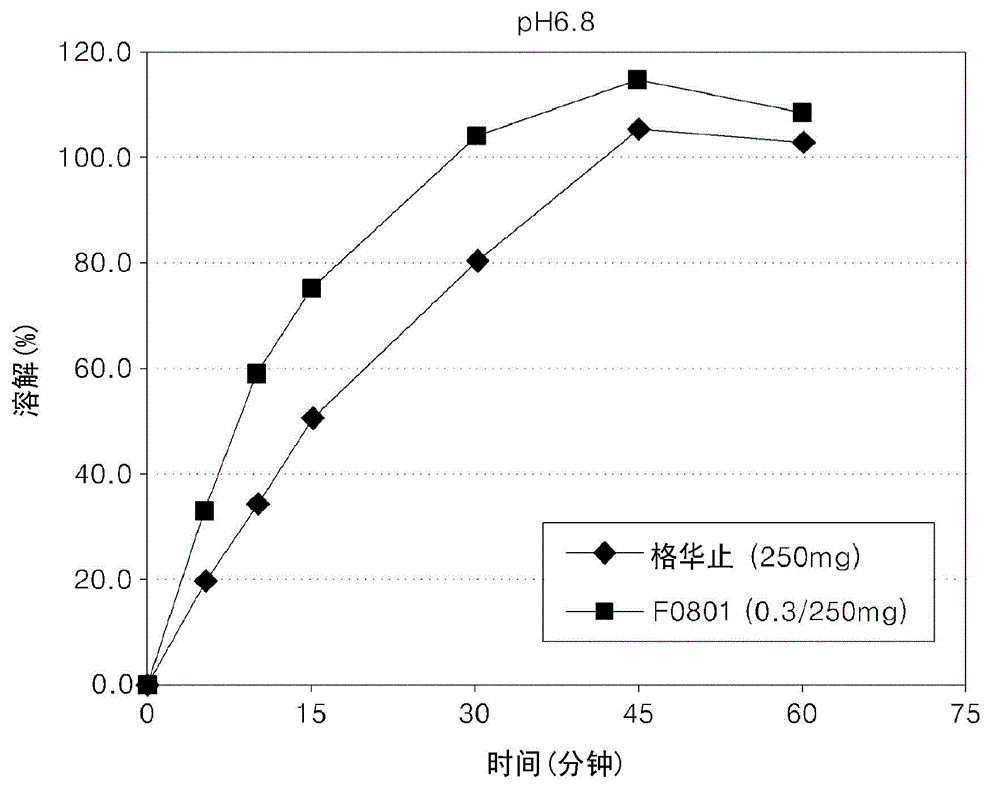
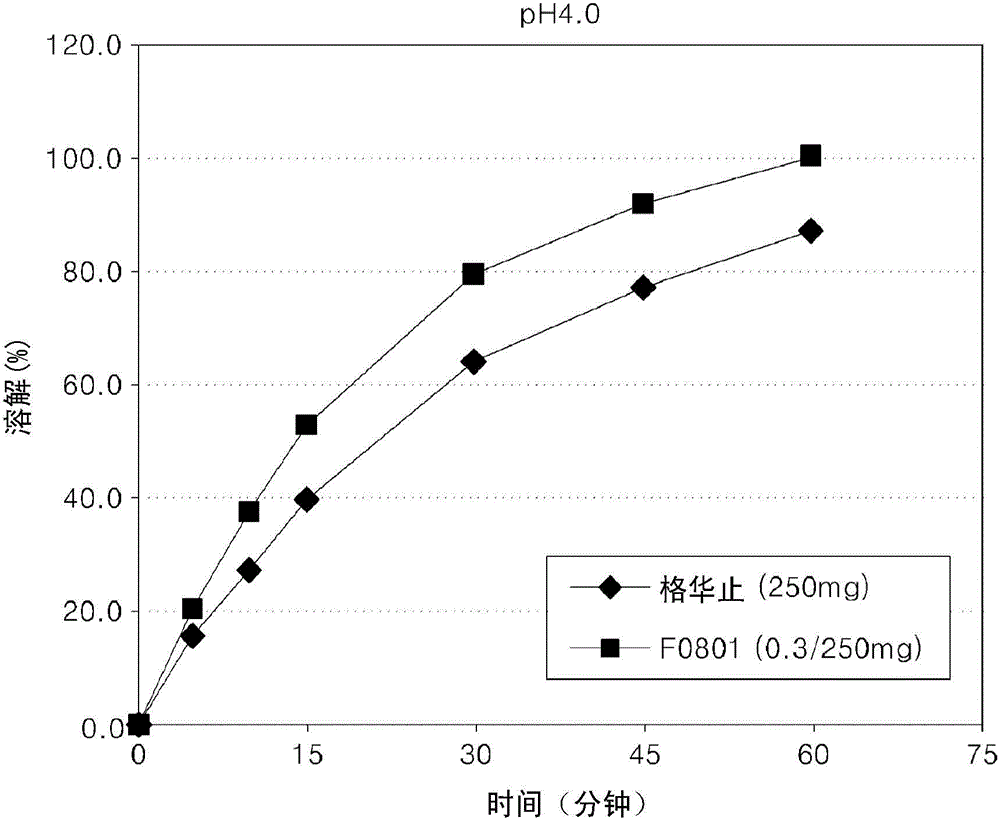
Oral formulations containing metformin and alpha-glycosidase inhibitor, and preparation method thereof
A technology of metformin and compound preparations, which is applied in the direction of medical preparations containing active ingredients, pill delivery, pharmaceutical formulations, etc. It can solve the problems of metformin not being released quickly and the bioavailability of metformin decrease, so as to control blood sugar and increase adaptability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of metformin and voglibose compound preparation (1)
[0044] 300g of metformin hydrochloride and 36g of hydroxypropyl cellulose were uniformly mixed; sieved with a 20-mesh mesh; added to a fluidized bed granulator; and then preheated at 60°C for 10 minutes. 6 g of hydroxypropyl cellulose was dissolved in 120 mL of water to prepare a binding solution; the binding solution was sprayed at a rate of 7 mL per minute, thereby preparing primary granules; and the primary granules were then sieved using a 20-mesh mesh.
[0045] 0.18 g of voglibose and 6 g of hydroxypropyl cellulose were dissolved in 120 mL of water by thorough stirring, and then fully sprayed onto the granules prepared in the above manner to prepare secondary granules. Finish by sieving the secondary granules using a 20 mesh mesh, then drying.
Embodiment 2
[0046] Example 2: Preparation of metformin and voglibose compound preparation (2)
[0047] 300g of metformin hydrochloride and 36g of hydroxypropyl cellulose were uniformly mixed; sieved with a 20-mesh mesh; added to a fluidized bed granulator; and then preheated at 60°C for 10 minutes. 6 g of hydroxypropyl cellulose was dissolved in 120 mL of water to prepare a binding solution; the binding solution was sprayed at a rate of 7 mL per minute, thereby preparing primary granules; and the primary granules were then sieved using a 20-mesh mesh.
[0048] 0.12 g of voglibose and 6 g of hydroxypropyl cellulose were dissolved in 120 mL of water by thorough stirring, and then sprayed onto the granules prepared in the above manner to prepare secondary granules. Finish by sieving the secondary granules using a 20 mesh mesh, then drying.
Embodiment 3
[0049] Example 3: Preparation of metformin and voglibose compound preparation (3)
[0050] 300g of metformin hydrochloride and 36g of hydroxypropyl cellulose were uniformly mixed; sieved with a 20-mesh mesh; added to a fluidized bed granulator; and then preheated at 60°C for 10 minutes. 6 g of hydroxypropyl cellulose was dissolved in 120 mL of water to prepare a binding solution; the binding solution was sprayed at a rate of 7 mL per minute, thereby preparing primary granules; and the primary granules were then sieved using a 20-mesh mesh.
[0051] 0.36 g of voglibose and 6 g of hydroxypropyl cellulose were dissolved in 120 mL of water by thorough stirring, and then sprayed onto the granules prepared in the above manner to prepare secondary granules. Sieve the secondary granules through the use of a 20-mesh mesh, then dry and finish.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com