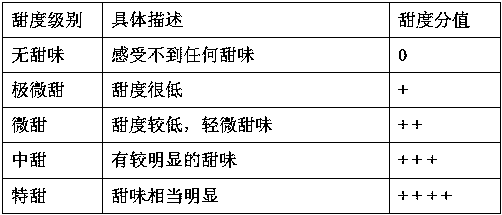
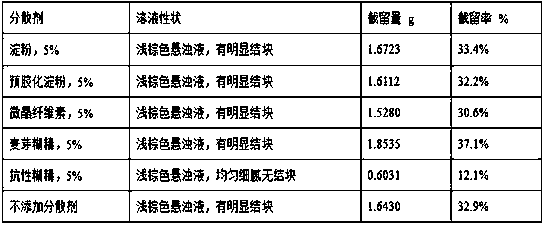
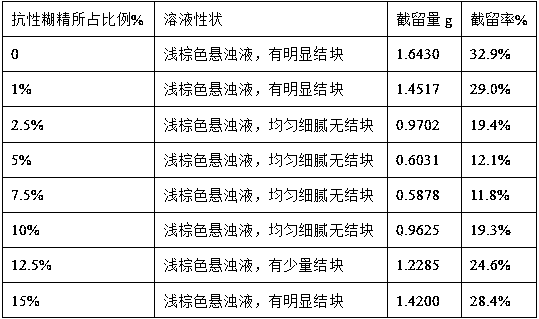
Oat beta-glucan oral composition and application thereof
A technology of glucan and composition, applied in the field of oral oat beta-glucan composition, can solve the problems of oat beta-glucan having poor taste, poor mouthfeel, inability to fully swell active ingredients, limited physical properties, and the like, Achieve the effect of significantly regulating blood lipids, reducing low-density lipoprotein and serum cholesterol, and evenly distributed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Raw material ratio (weight percent):
[0036] Oat beta-glucan: 85%
[0037] Resistant Dextrin: 5%
[0038] Fructose-oligosaccharides: 10%.
[0039] Preparation:
[0040] Mix the three components evenly.
Embodiment 2
[0042] Raw material ratio (weight percent):
[0043] Oat beta-glucan: 87.5%
[0044] Resistant Dextrin: 7.5%
[0045] Fructooligosaccharides: 5%.
Embodiment 3
[0047] Raw material ratio (weight percent):
[0048] Oat beta-glucan: 75%
[0049] Resistant Dextrin: 10%
[0050] Fructooligosaccharides: 15%.
[0051] The beneficial effect of the prescription of the present invention is further illustrated by human body test data.
[0052] 1. Blood lipid-lowering effect test:
[0053] 1. Subject selection: 30 patients with hyperlipidemia who meet the following criteria:
[0054] (1) Age 32~74 years old, gender is not limited;
[0055] (2) The body mass index is within the range of 19~30kg / m2;
[0056] (3) The blood low-density lipoprotein cholesterol (LDL-C) level is between 3.38 mmol / L and 4.52 mmol / L;
[0057](4) Without lipid-lowering drug treatment or lipid-lowering drug withdrawal for 12 weeks;
[0058] (5) Pregnant women, breastfeeding women, those taking hormonal drugs, patients with severe chronic diseases, and patients in the withdrawal period after surgery were excluded.
[0059] Using a randomized parallel controlled tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com