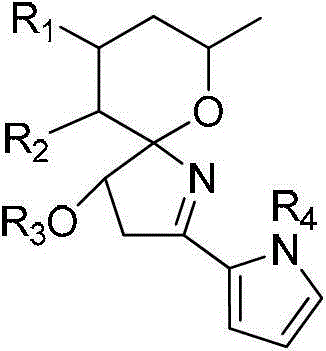
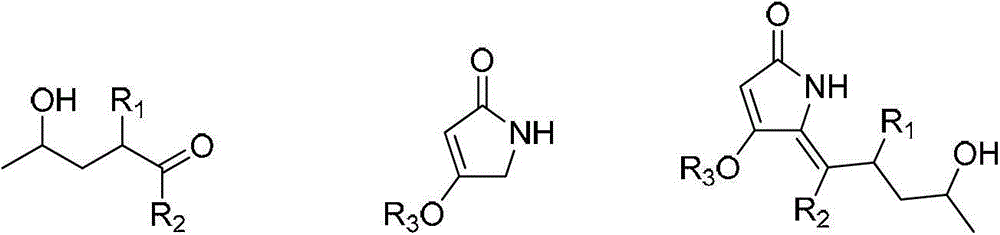
Preparation method of prodigiosin derivative
A technology for prodigiosin and derivatives, applied in the field of preparation of prodigiosin derivatives, capable of solving problems such as low yield and single product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, the preparation of prodigiosin derivative
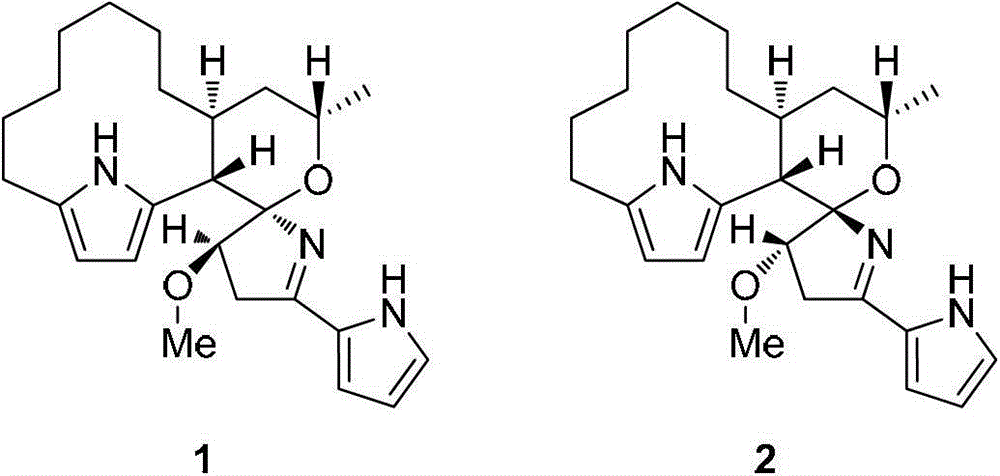
[0048] The structural formula of the prepared prodigiosin derivative is shown in formula I-a.
[0049]
[0050] (Formula IV-a) (Formula III-a) (Formula V-a)
[0051]
[0052] (Formula VI-a) (Formula VII-a) (Formula I-a)
[0053] Dissolve 8.40 g of the compound represented by formula III-a in 110 mL of 4N sodium hydroxide solution and heat to 55°C. Dissolve 6.90 g of the compound represented by formula II-a in 110 mL of methanol, then drop this solution into the above-mentioned sodium hydroxide solution, and allow it to react at 55° C. for 12 hours after the addition is complete. Cool to room temperature, concentrate to remove the organic solvent, and then put it in the refrigerator (0° C.) for 24 hours to precipitate a white solid. After filtration and washing with water, 7.28 g of the compound represented by formula IV-a was obtained as a white solid (55%).
[0054] The structure confirmation results a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com