Preparation method of fluorescent waterborne polyurethane emulsion based on chromophore in diisocyanate
A technology of diisocyanate and water-based polyurethane, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of color variety, fluorescent function, vividness, migration resistance and weather resistance, and insufficient content of fluorescent conjugated groups control, complex reaction and other issues, to achieve the effect of good fluorescence intensity persistence, not easy to migrate, and uniform distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
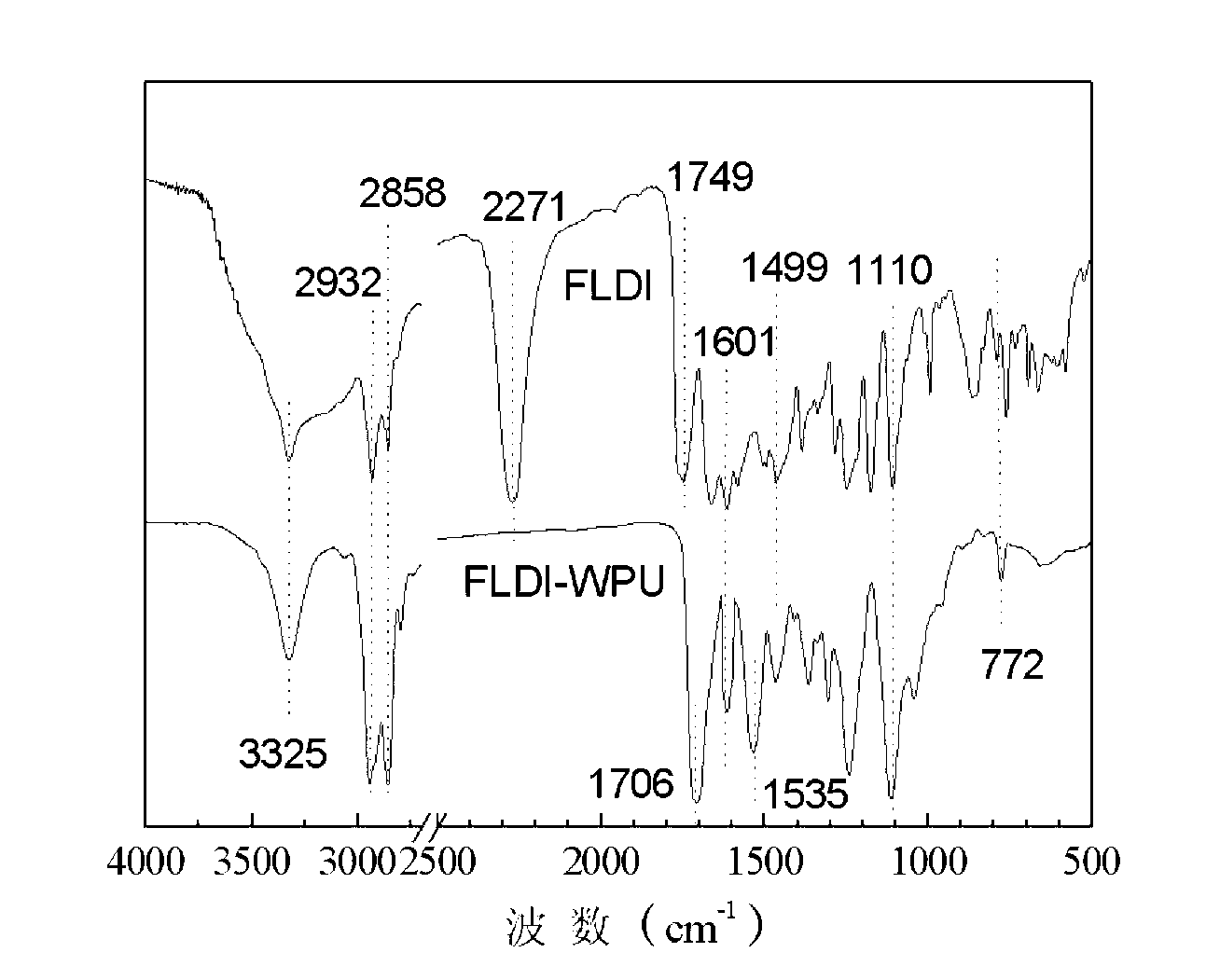
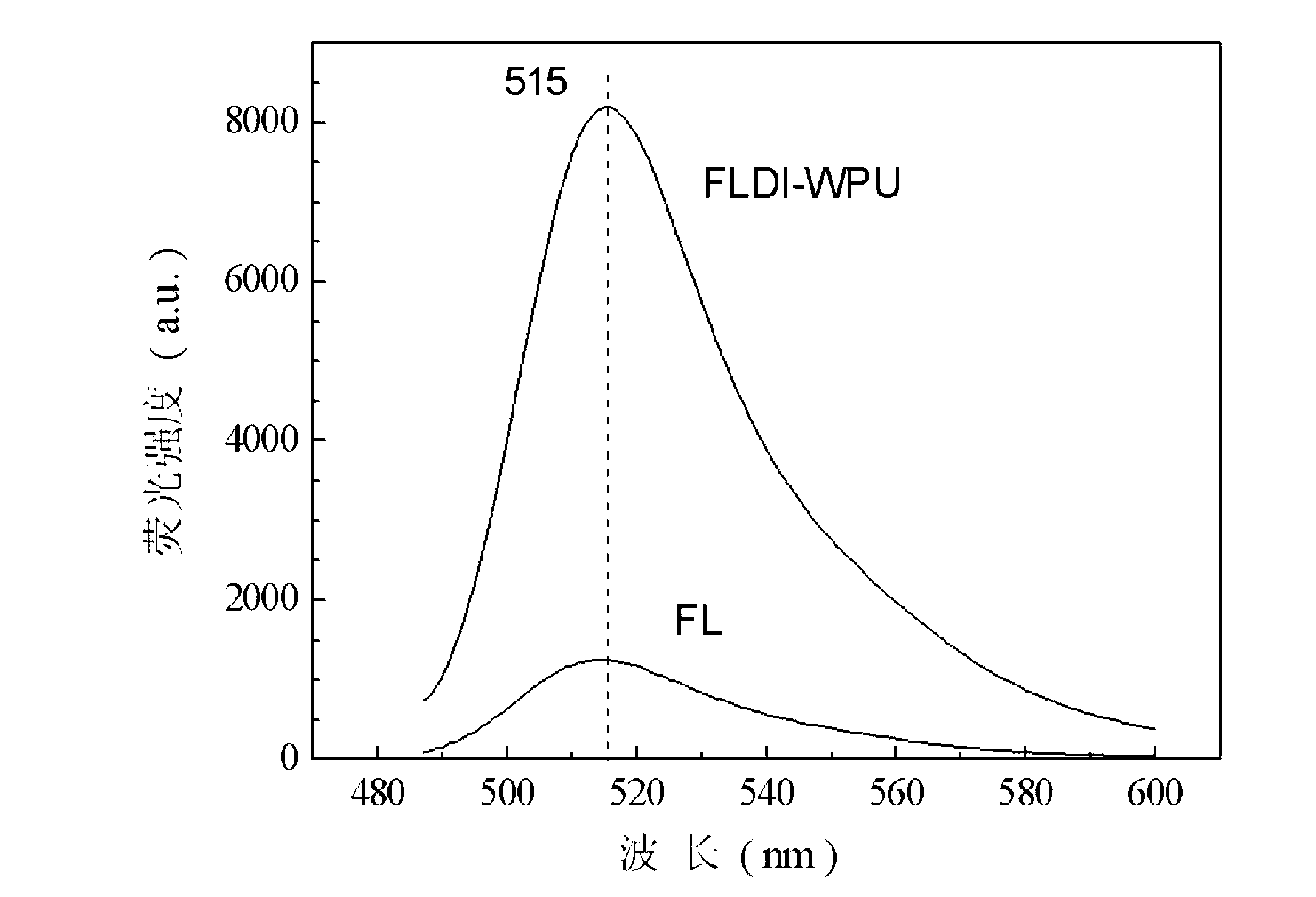
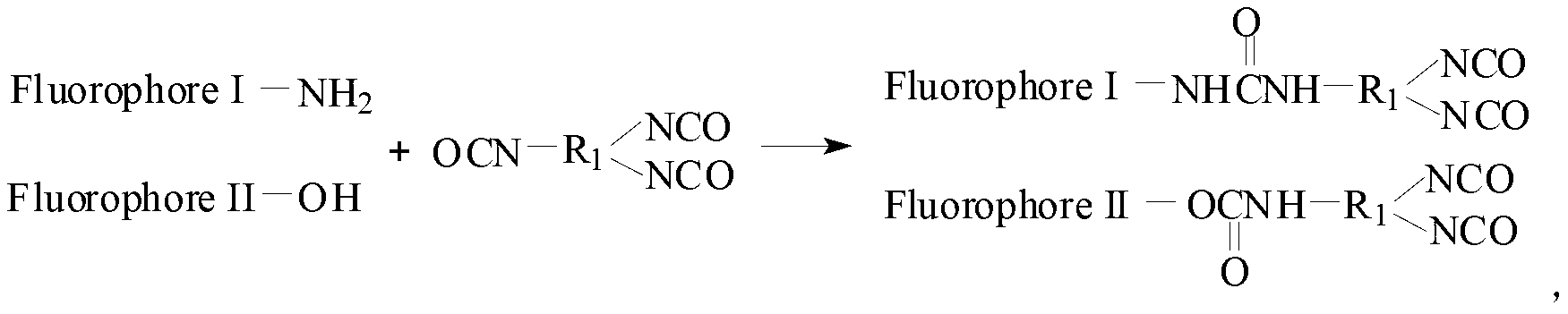
[0047] Add 10.00 grams of HDI and 9.88 grams of FL into a three-necked flask, add 60 milliliters of N,N-dimethylformamide as a solvent, and then add 0.01 grams of DBTDL as a catalyst, react at 60°C for 5 hours under mechanical stirring, and cool to 30 The solvent was distilled off under reduced pressure at ℃, and then the product was washed with acetone for 3 times, and the washed product was placed in a vacuum oven and dried to constant weight to obtain fluorescent diisocyanate FLDI.
[0048] 36.00 g of PTMG (M n =2000) into a 500ml three-necked flask, dehydrated at 115°C for 1 hour and then cooled to 50°C. Take 7.95 grams of fluorescent diisocyanate FLDI and 15.60 grams of IPDI into a three-necked flask, stir and react at 85°C for 3 hours, then add 3.70 grams of hydrophilic chain extender DMPA, 2.80 grams of BDO, 0.02 grams of DBTDL and 45.00 grams of methyl ethyl ketone. Stir and react at 75°C for 3 hours, then cool down to 30°C, transfer the reaction product to a high-spe...
Embodiment 2
[0053] Add 6.00 g of p-phenylene diisocyanate and 20 ml of N,N-dimethylformamide into a three-necked flask, raise the temperature to 30°C, add ST6.60 g and 30 ml of N,N-dimethylformamide dropwise under mechanical stirring The solution is controlled to be added dropwise in half an hour. After keeping the reaction at 30°C for 2 hours, the solvent was distilled off under reduced pressure, and the product was washed once with dichloromethane. The washed product was placed in a vacuum drying oven and dried to constant weight to obtain fluorescent diisocyanate STDI.
[0054] Add 10.00 g of HDI trimer and 20 ml of N,N-dimethylformamide into a three-necked flask, raise the temperature to 30°C, add 2.84 g of AN and 6 ml of N,N-dimethylformamide dropwise under mechanical stirring Solution, control the dropwise addition in half an hour. After keeping the reaction at 30°C for 2 hours, the solvent was distilled off under reduced pressure, and the product was washed once with dichlorometha...
Embodiment 3
[0058] Add 25.00 grams of HDI trimer and 30 milliliters of N,N-dimethylformamide into a 250-mL three-necked flask, raise the temperature to 30 ° C, protect with nitrogen, and add 4.50 grams of Azure A1 and 30 milliliters of N , The solution of N-dimethylformamide was added dropwise in half an hour. After keeping the reaction at 30°C for 2 hours, the solvent was distilled off under reduced pressure, and the product was washed once with acetone. The washed product was placed in a vacuum drying oven and dried to constant weight to obtain fluorescent diisocyanate AADI.
[0059] 60.00 grams of PPG (M n =1000) into a 500mL three-necked flask, dehydrated at 120°C for 1 hour and then cooled to 50°C. Take 18.00 grams of fluorescent diisocyanate AADI, 35.00 grams of TDI, and 0.03 grams of DBTDL into a three-necked flask, stir and react at 80°C for 3 hours, then add 7.20 grams of hydrophilic chain extender DMPA, 8.60 grams of DEG and 90.00 grams of acetone , constant temperature was st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com