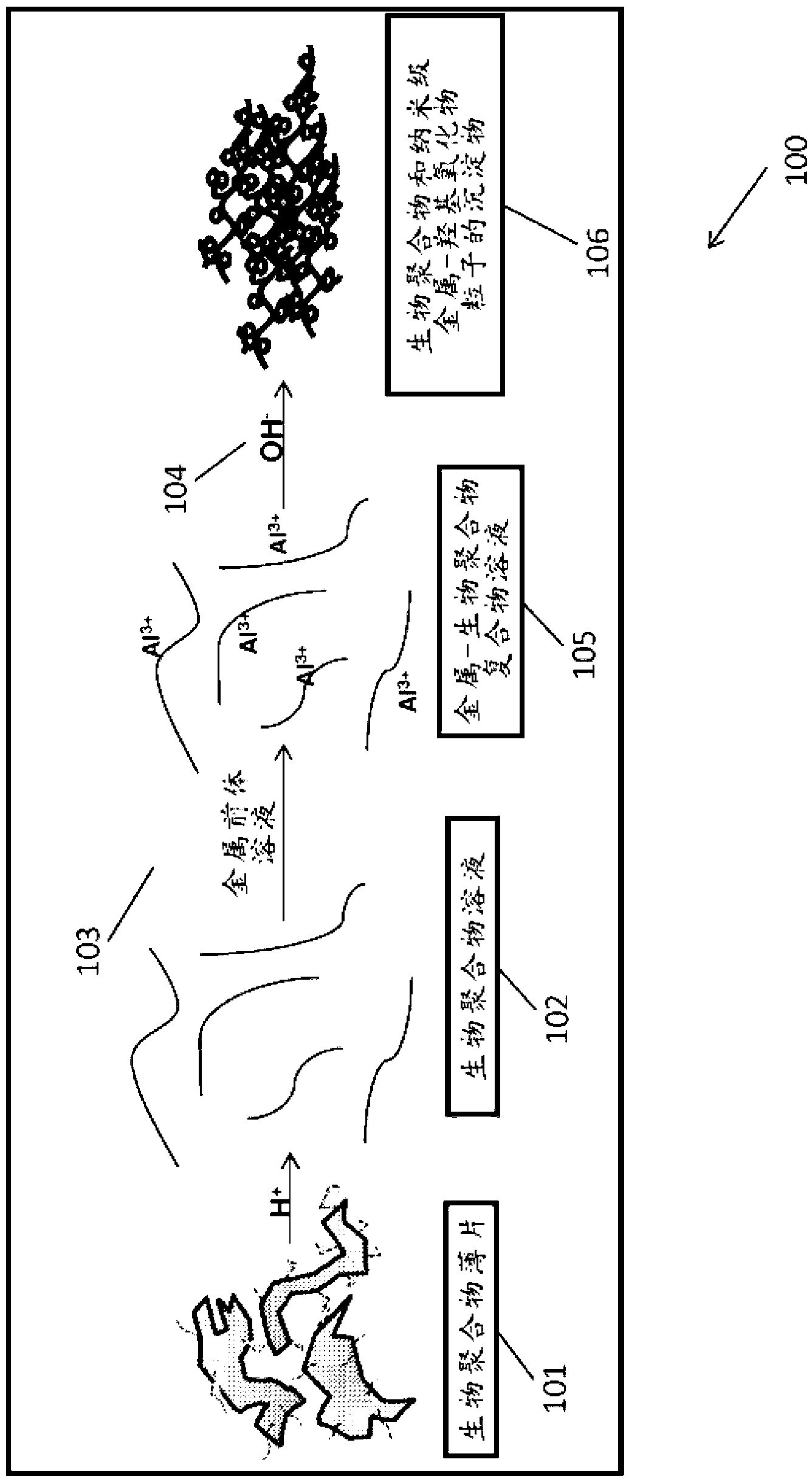
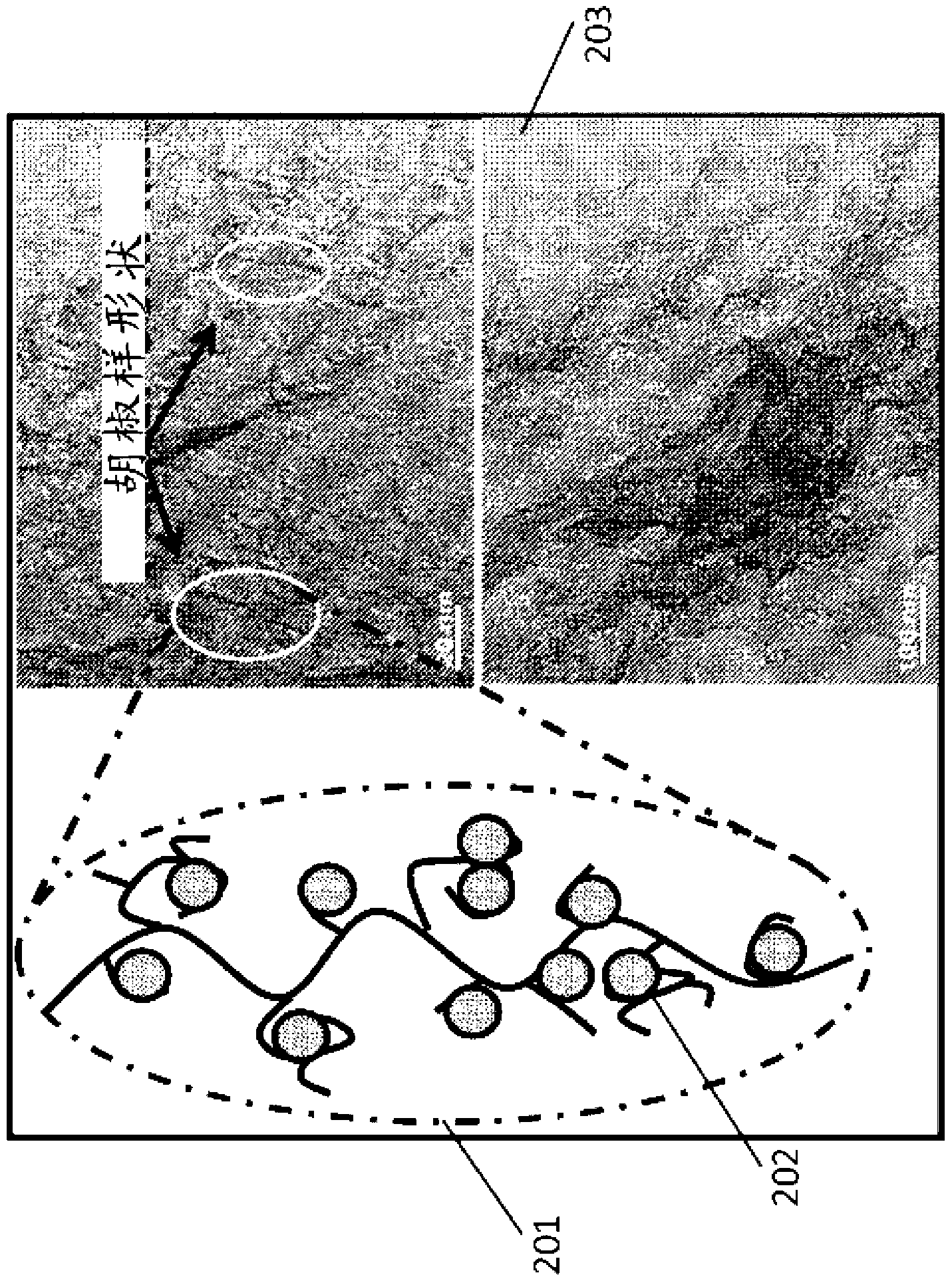
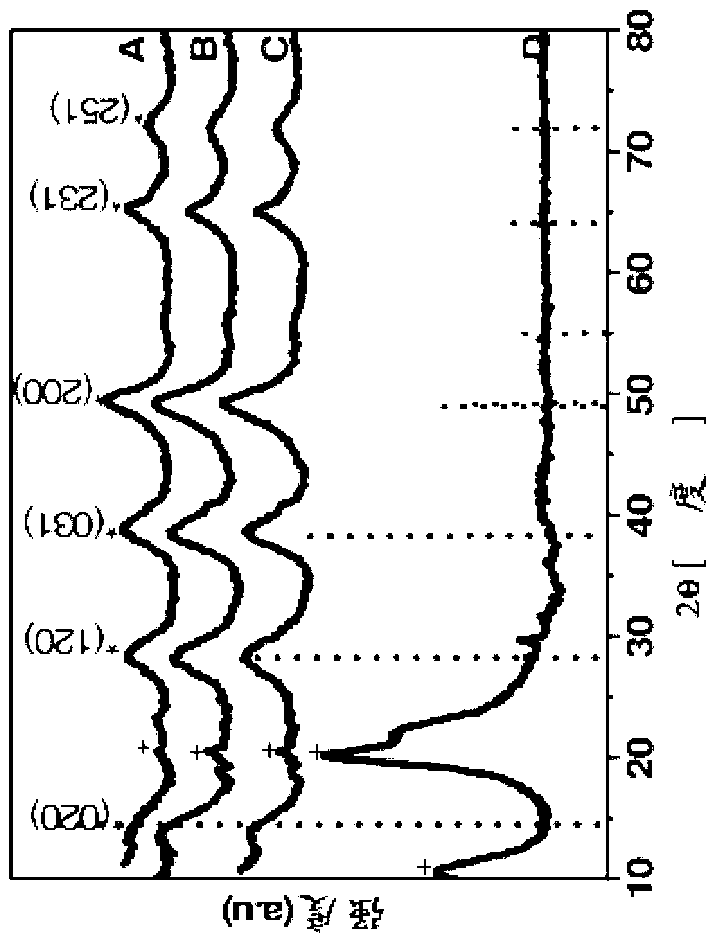
Organic templated nanometal oxyhydroxide
An oxyhydroxide and nano-metal technology, applied in the field of nano-materials, can solve the problems of decomposition of chitosan, reduction of fluoride intake capacity, clogging of filter devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077]This example describes the low-temperature synthesis of nanoscale-AlOOH via a simple and mild chemical route. This synthesis method involves mixing an aluminum precursor solution with chitosan (dissolved in 1-5% glacial acetic acid or HCl or a combination thereof) with vigorous stirring. In a general method, a solution of an aluminum precursor such as aluminum nitrate is slowly added to a chitosan solution with vigorous stirring for 60 minutes and left overnight without stirring. Ammonia or NaOH solution was slowly added to the metal-chitosan solution with vigorous stirring to promote the precipitation of the metal-chitosan composite material (pH7-8.0). All these steps are carried out at a temperature below 30°C. Stirring was continued for 2 hours. The precipitate was filtered, washed to remove any unwanted impurities, converted to bead shape and dried under various conditions.
Embodiment 2
[0079] This example describes the preparation of OTBN using other biopolymers via a simple and mild chemical route. This synthesis method involves mixing an aluminum precursor solution with cellulose with vigorous stirring. In a general procedure, a solution of an aluminum precursor such as aluminum nitrate is slowly added to a polymer solution with vigorous stirring for 60 minutes and left overnight without stirring. Ammonia or NaOH solution was slowly added to the metal-cellulose solution with vigorous stirring to facilitate the precipitation of the metal-cellulose composite (pH 7-8.0). All these steps are carried out at a temperature below 30°C. Stirring was continued for 2 hours. The precipitate was filtered, washed to remove any unwanted impurities, converted to bead shape and dried under various conditions.
Embodiment 3
[0081] This example describes the preparation of OTBN by a simple and mild chemical route using a mixture of biopolymers. The biopolymers used in this study were chitosan and cellulose. Cellulose powder was added to the chitosan solution (chitosan dissolved in 1% acetic acid). The weight ratio of chitosan to cellulose is 1:1. Additionally, the aluminum nitrate solution was slowly added to the biopolymer solution with vigorous stirring for 60 minutes and left overnight without stirring. Ammonia or NaOH solution was slowly added to the metal-chitosan solution with vigorous stirring to promote the precipitation of the metal-cellulose-chitosan composite material (pH7-8.0). All these steps are carried out at a temperature below 30°C. Stirring was continued for 2 hours. The precipitate was filtered, washed to remove any unwanted impurities, converted to bead shape and dried under various conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com