transdermal preparation
A technology for transdermal absorption preparations and absorption accelerators, which is applied in the direction of antifungal agents, aerosol delivery, medical preparations of non-active ingredients, etc., and can solve the problems that it is difficult to provide transdermal absorption and difficult to pass through membranes. , to achieve excellent transdermal properties, reduce burden, and improve medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
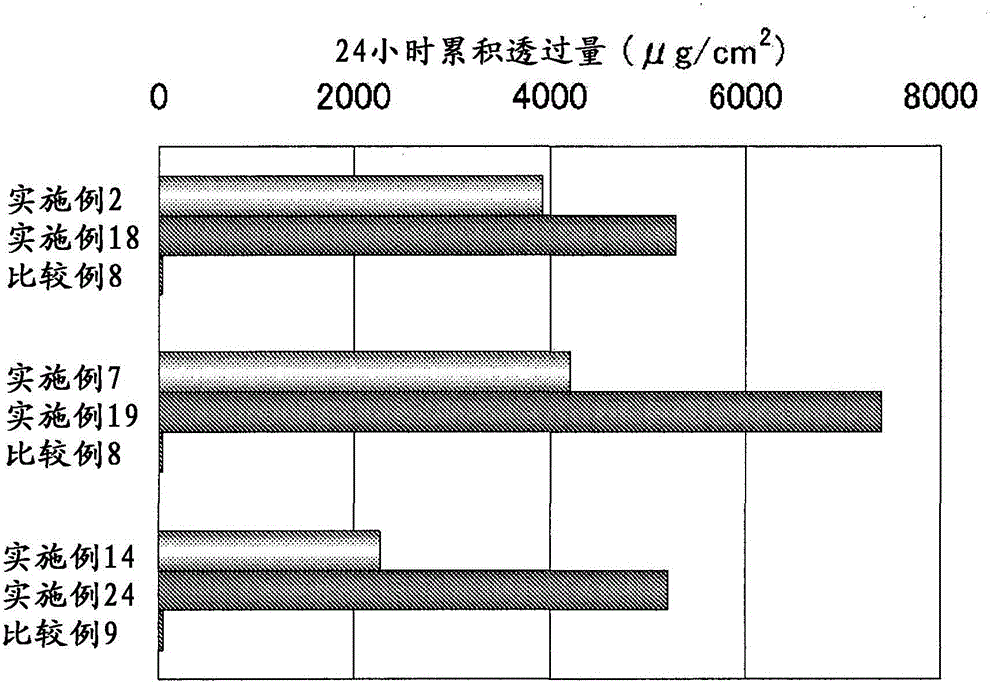
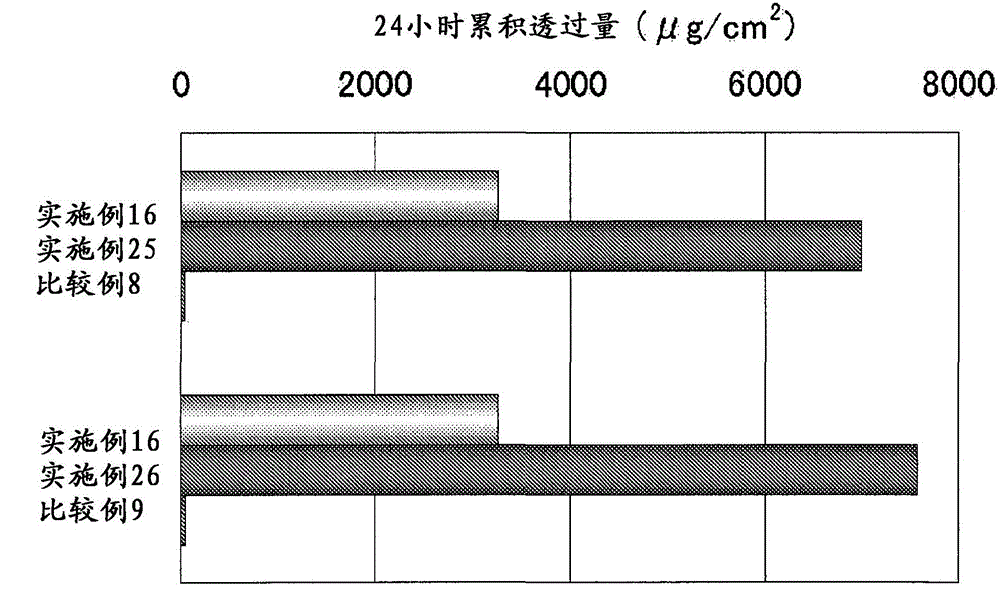
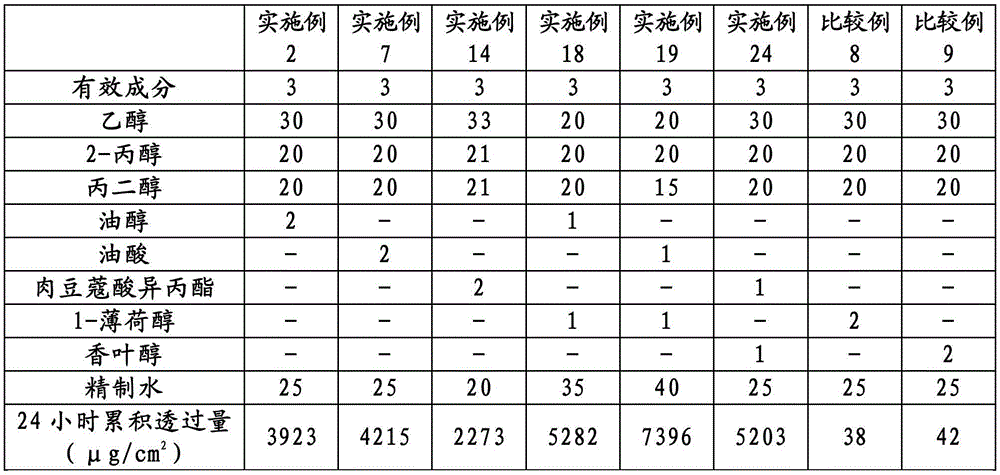
[0062] 3 parts of active ingredients, 5 parts of oleyl alcohol, 10 parts of propylene glycol, 70 parts of ethanol and 12 parts of purified water were mixed to obtain 100 parts of the preparation.
Embodiment 2
[0064] 3 parts of active ingredients, 2 parts of oleyl alcohol, 20 parts of propylene glycol, 20 parts of 2-propanol, 30 parts of ethanol and 25 parts of purified water were mixed to obtain 100 parts of the preparation.
Embodiment 3
[0066] 3 parts of active ingredients, 5 parts of octanoic acid, 10 parts of propylene glycol, 70 parts of ethanol and 12 parts of purified water were mixed to obtain 100 parts of the preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com