Daphnetin slow-release composition and preparation method thereof
A slow-release composition and technology of daphnetin, applied in drug combination, active ingredient of heterocyclic compounds, drug delivery, etc., can solve the problems of affecting the therapeutic effect of daphnetin, poor patient compliance, low bioavailability, etc., to reduce medication The effect of frequency, delayed drug release, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The preparation of embodiment 1 Daphnetin Sustained-release Tablets
[0067] Daphnetin Sustained Release Tablets Prescription Composition:
[0068]
[0069]
[0070] Preparation method: Weigh the prescribed amount of daphnetin, starch, hydroxypropylmethylcellulose, and polyvinylpyrrolidone, mix them and pass through an 80-mesh sieve, use 90% ethanol as a wetting agent to make a soft material, make wet granules, dry, add Magnesium stearate, mixed evenly, granulated, compressed into tablets, ready to be obtained.
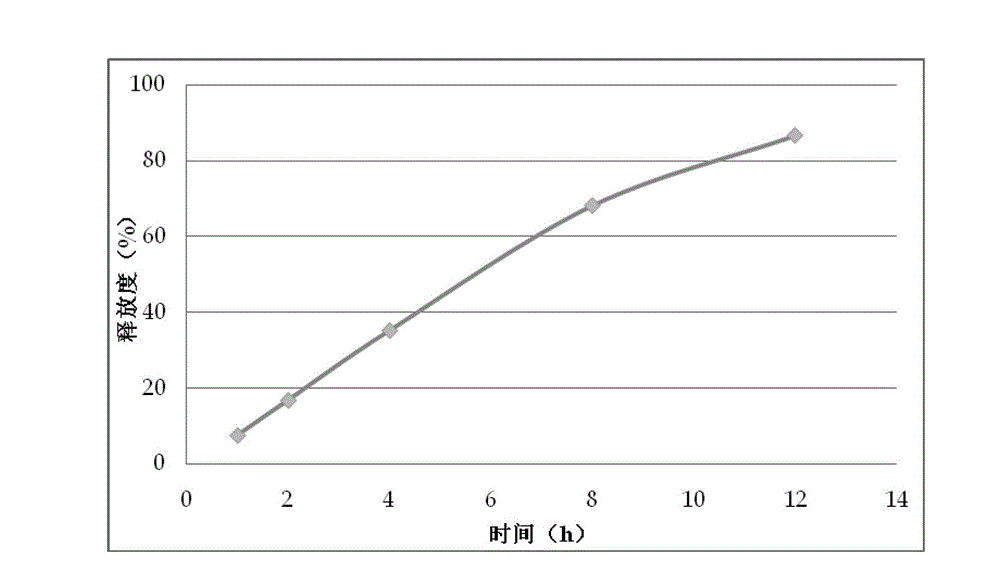
[0071] Its in vitro release curve is shown in figure 1 .
Embodiment 2
[0072] The preparation of embodiment 2 daphnetin sustained-release tablets
[0073] Daphnetin Sustained Release Tablets Prescription Composition:
[0074]
[0075] Preparation method: Weigh the prescribed amount of daphnetin, starch, hydroxypropylmethylcellulose, and polyvinylpyrrolidone, mix them evenly and pass through an 80-mesh sieve, use 5% ethanol solution of polyvinylpyrrolidone as a binder to make a soft material, and make wet Granules, dried, added with magnesium stearate, mixed evenly, granulated, compressed into tablets, to obtain.
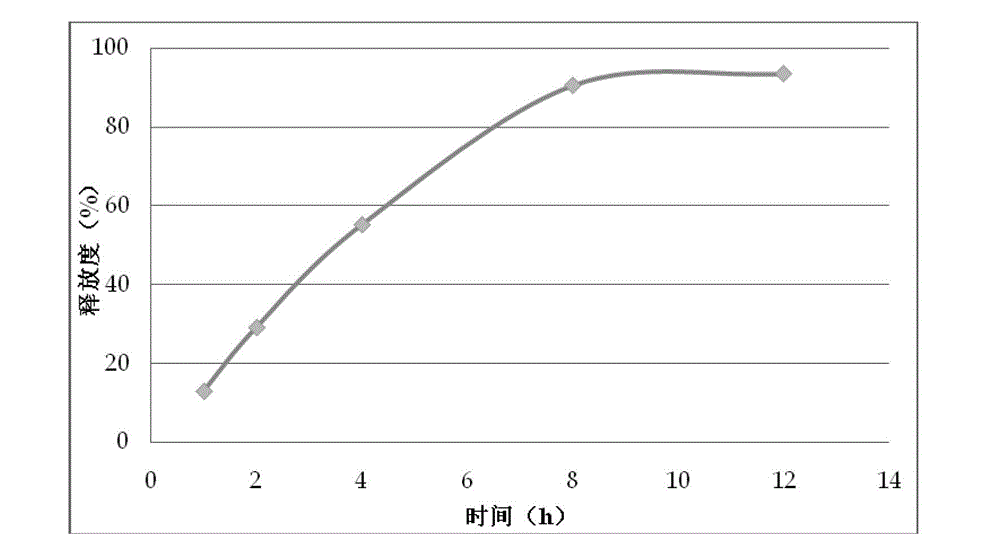
[0076] Its in vitro release curve is shown in figure 2 .
Embodiment 3
[0077] The preparation of embodiment 3 daphnetin sustained-release tablets
[0078] Daphnetin Sustained Release Tablets Prescription Composition:
[0079]
[0080]
[0081] Preparation method: Weigh the prescribed amount of daphnetin, starch, hydroxypropylmethylcellulose, and polyvinylpyrrolidone, mix them and pass through an 80-mesh sieve, use 15% ethanol solution of polyvinylpyrrolidone as a binder to make a soft material, and make wet Granules, dried, added with magnesium stearate, mixed evenly, granulated, compressed into tablets, to obtain.
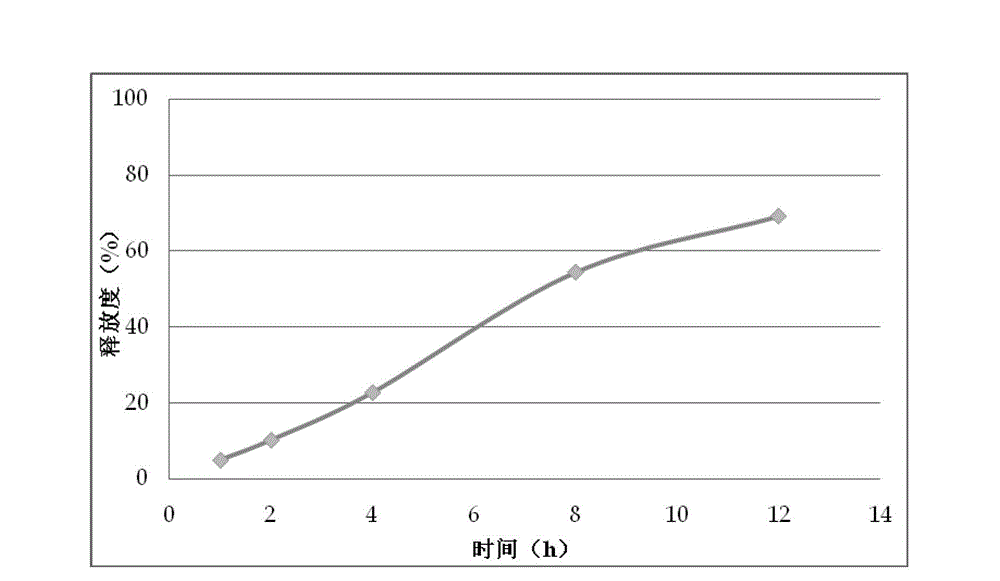
[0082] Its in vitro release curve is shown in image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com