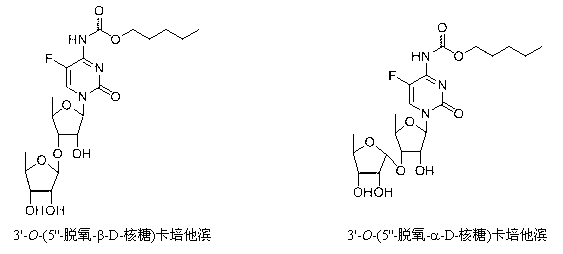
Preparation method of 5-deoxy-D-ribofuranose oxygen glycosides compound
A technology of ribofuranosides and compounds, which is applied to the preparation of sugar derivatives, chemical instruments and methods, and sugar derivatives, and can solve problems such as unstable quality control and difficult implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
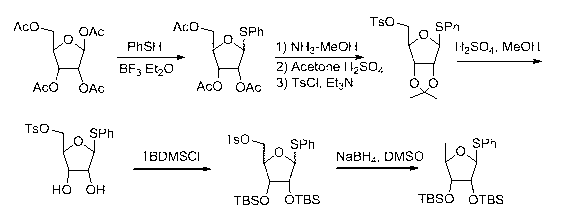
[0030] Example 1 2,3-two- O -Synthesis of tert-butyldimethylsilyl-5-deoxy-β-D-ribofuranose glucosinolate sugar donor
[0031] Dissolve 16g of tetraacetyl ribose and 6g of thiophenol in 100mL of dichloromethane, add BF dropwise under ice bath 3 -Et 2 O 2mL, after the addition was completed, the ice bath was removed to react for 4 hours, the reaction solution was washed with saturated sodium bicarbonate until neutral, the organic layer was separated and concentrated to obtain a yellowish oil. Disperse the oily substance in 250 mL of methanol, pass ammonia gas to saturation, react for 12 hours, evaporate the solvent under reduced pressure, and obtain a light yellow oily substance. Disperse the oil in 200mL of acetone, slowly add 1mL of concentrated sulfuric acid dropwise, and react at room temperature for 12 hours, add 10g of sodium bicarbonate, stir until the reaction solution is weakly alkaline, concentrate under reduced pressure, and dissolve the obtained residue in 200m...
Embodiment 2
[0033] Example 2 2'- O -Synthesis of tert-butyldimethylsilyl capecitabine
[0034] Take 3.6 g of capecitabine, dissolve it in 100 mL of anhydrous dichloromethane, add 1 g of imidazole and 2 g of tert-butyldimethylchlorosilane, stir for 1 hour, add water to terminate the reaction, separate the organic layer, and obtain 2'- O - tert-butyldimethylsilyl capecitabine 2.5g, yield 52.7%.
Embodiment 3
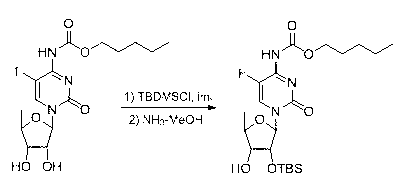
[0035] Example 3 3'-(5''-deoxy-2'',3''-di- O -tert-butyldimethylsilyl-β-D-ribose)-2'- O -tert-butyldimethylsilyl capecitabine and 3'-(5''-deoxy-2,3-di- O -tert-butyldimethylsilyl-α-D-ribose)-3'- O -Synthesis of tert-butyldimethylsilyl capecitabine
[0036] Take 2'- O - tert-Butyldimethylsilyl capecitabine 473 mg, 2,3-di- O - tert-butyldimethylsilyl-5-deoxy-β-D-ribofuranose glucosinolate sugar donor 680mg, N - Dissolve 520 mg of iodosuccinimide in anhydrous dichloromethane, add molecular sieves, stir for 2 hours under argon protection, add 100 mg of silver trifluoromethanesulfonate, continue stirring for 2 hours, add saturated sodium thiosulfate solution, Stir for 10 minutes, separate the layers, and perform column chromatography to obtain 3'-(5''-deoxy-2'',3''-di- O -tert-butyldimethylsilyl-β-D-ribose)-2'- O - tert-butyldimethylsilyl capecitabine 460mg, yield 56.3%; 3'-(5''-deoxy-2,3-di- O -tert-butyldimethylsilyl-α-D-ribose)-3'- O - tert-butyldimethylsilyl capec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com