Method for recovering rare earth, thorium and iron in waste residue of rare earth acid technological process
A recovery method and rare earth technology, applied in the direction of improving process efficiency, etc., to achieve the effect of solving radioactive waste residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: by 0.4:1 acid slag ratio with 20.0g water immersion waste residue and acidity be the HNO of 3.0mol / L 3 The solution was mixed and placed in a beaker, covered with a glass, and kept stirring at 80°C for 1 hour for leaching. Filtrate hot, wash the filter cake three times, and combine the filtrate and washing liquid. The filter cake was dried at 100°C to obtain 9.7g secondary waste residue, ThO 2 Dissolution rate is 95.0%, REO dissolution rate is 51.2%, Fe 2 o 3 The dissolution rate is 86.3%, and the radioactive specific activity of the secondary waste residue is less than 7.4×10 4 Bq / Kg. The combined solution was extracted with primary amine, and back-extracted with nitric acid to form a thorium nitrate solution. The remaining liquid NaOH adjusts the pH to 4.5 to form Fe(OH) 3 By-products, the filtrate is rare earth feed liquid.
Embodiment 2
[0017] Example 2: Mix 20.0 g of water leaching waste residue with HCl solution with an acidity of 2.0 mol / L according to the ratio of 0.8:1 to acid residue, put it in a beaker, cover it with a watch glass, and keep stirring at 95°C for 1 hour for leaching. Filtrate hot, wash the filter cake three times, and combine the filtrate and washing liquid. The filter cake was dried at 100°C to obtain 9.0 g of secondary waste residue, ThO 2 Dissolution rate is 99.1%, REO dissolution rate is 62.1%, Fe 2 o 3 The dissolution rate is 88.0%, and the radioactive specific activity of the secondary waste residue is less than 7.4×10 4 Bq / Kg. The combined solution was extracted with primary amine, and back-extracted with nitric acid to form a thorium nitrate solution. residual liquid NH 3 ﹒ h 2 O adjusts the pH to 5.0 and forms crude Fe(OH) 3 The product, the filtrate is rare earth material liquid.
Embodiment 3~7
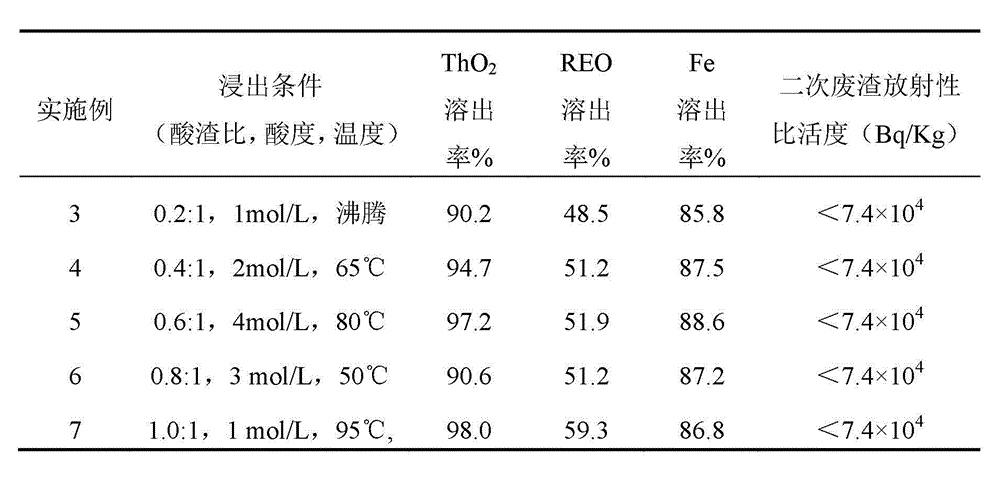
[0018] Embodiment 3 ~ 7: according to the ratio of acid slag, waste residue and different concentration of H 2 SO 4 The solutions were mixed, heated and stirred for leaching for 2 hours. Table 2 is ThO under different leaching conditions 2 , REO and Fe dissolution rate results. The leaching solution is extracted with primary amine, and back-extracted with nitric acid to form a thorium nitrate solution. The remaining liquid MgO adjusts the pH to 5.2 to form crude Fe(OH) 3 The product, the filtrate is rare earth material liquid.
[0019] Table 2 Example 3 ~ 7 leaching conditions and results
[0020]
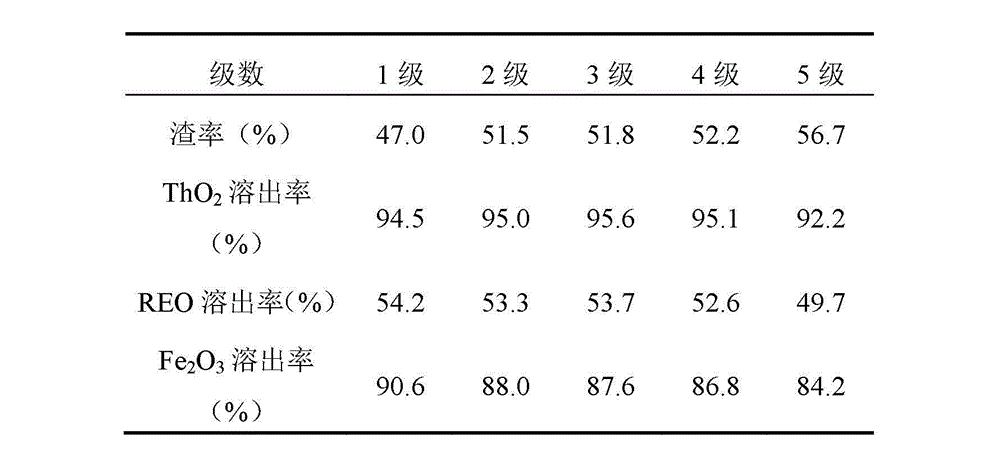
[0021] 8. Leaching liquid circulation leaching: Take about 20.0g of water leaching waste residue sample, add H with an acidity of 2 mol / L according to the acid residue ratio of 0.6:1 2 SO 4 130ml of the solution, covered with a surface dish, leaching at 80°C for 1 hour. Filtration, the filtrate is a pickling solution. Rinse the filter cake three times and discard the wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com