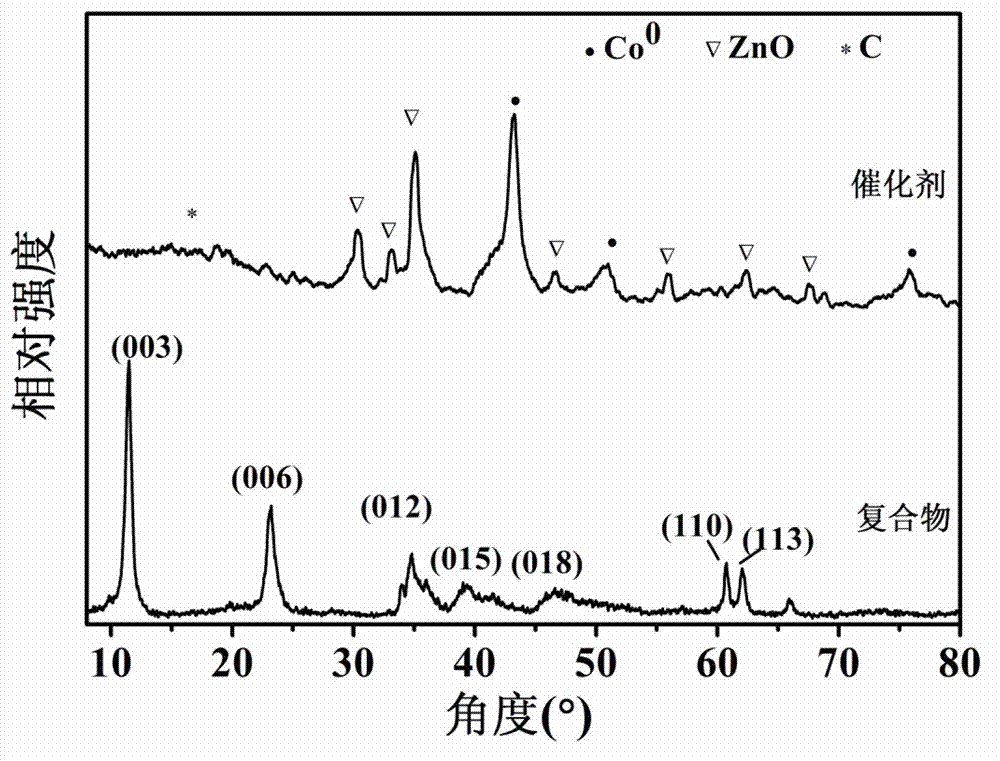
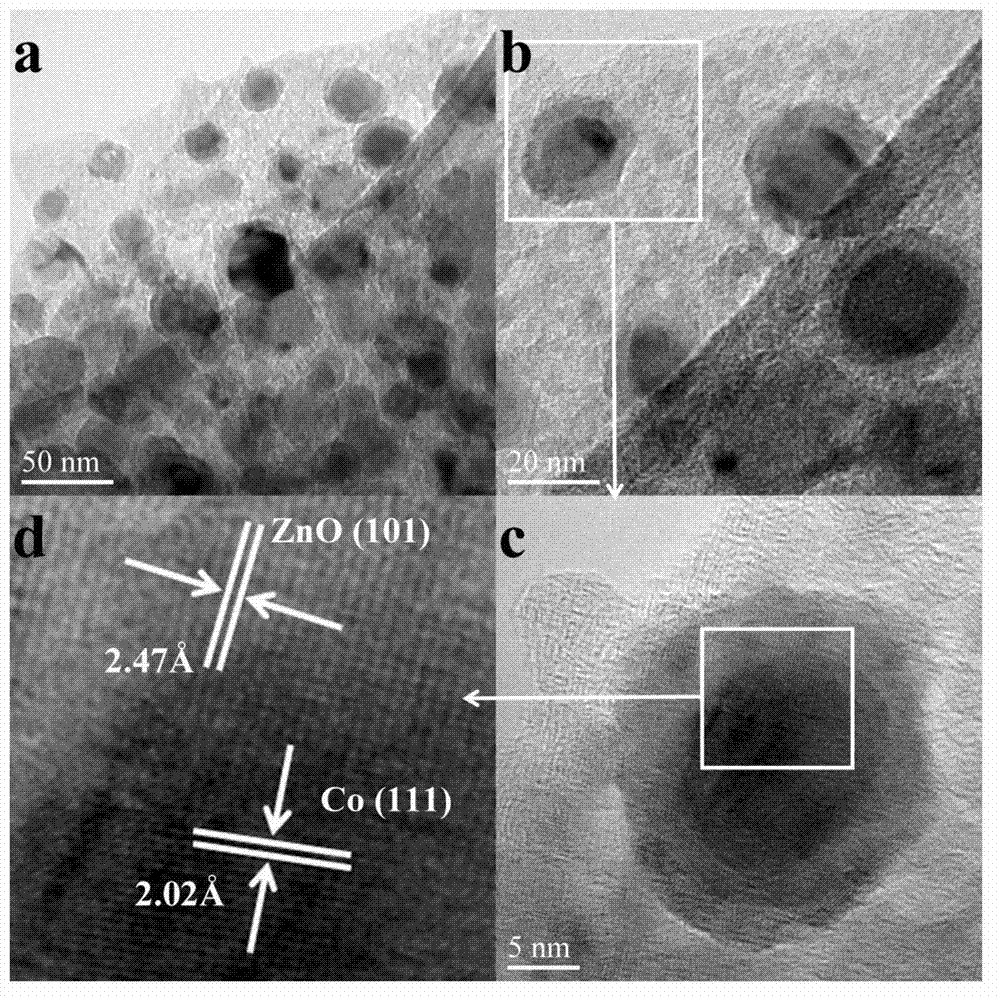
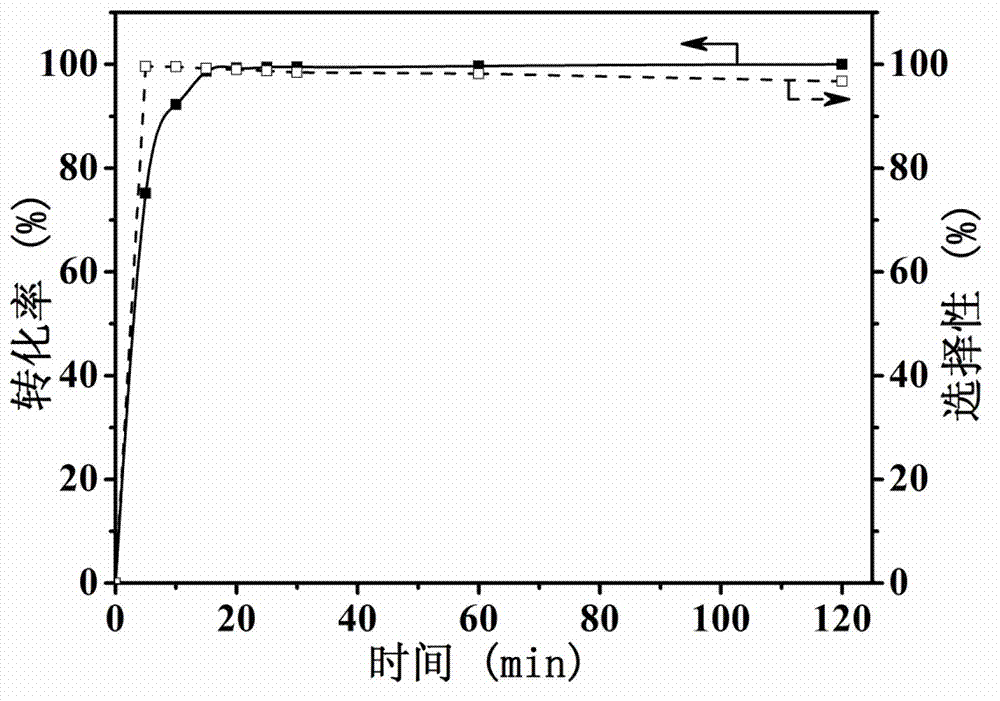
Carbon supported core-shell structure nano metal catalyst as well as preparation method and application thereof
A core-shell structure, nano-metal technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, preparation of hydroxyl compounds, etc. In order to achieve the effect of good catalytic hydrogenation performance, improved structural stability, and improved chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 0.006mol (CH 3 COO) 2 Co 4H 2 O, 0.006mol (CH 3 COO) 2 Zn·2H2 O, 0.003mol Al(NO 3 ) 3 9H 2 O was dissolved in 30ml deionized water as solution A. Another 0.024mol NaOH, 0.0075mol CH 3 COONa was dissolved in 30ml deionized water as solution B. Slowly pour solution A and solution B into the colloid mill (4000rpm), fully stir and mix for 3 minutes, centrifuge the slurry and wash it 3 times to make it neutral, fully disperse the slurry in 60ml deionized water, transfer to 80ml poly Crystallized at 140°C for 8 hours in a vinyl fluoride reactor, then centrifuged and washed to obtain CoZnAl-LDH. The obtained CoZnAl-LDH slurry and 0.00225mol β-cyclodextrin were dispersed in 100ml deionized water at 80°C and stirred until all the water evaporated to obtain a CoZnAl-LDH / β-cyclodextrin complex.
[0022] The CoZnAl-LDH / β-cyclodextrin complex prepared above was placed in a tube atmosphere furnace under N 2 Under the atmosphere (60mL / min), the temperature was raised to 200...
Embodiment 2
[0025] 0.009mol (CH 3 COO) 2 Co 4H 2 O, 0.003mol (CH 3 COO) 2 Zn·2H 2 O, 0.003mol Al(NO 3 ) 3 9H 2 O was dissolved in 30ml deionized water as solution A. Another 0.02mol NaOH, 0.005mol CH 3 COONa was dissolved in 30ml deionized water as solution B. Slowly pour solution A and solution B into the colloid mill (4000rpm), fully stir and mix for 3 minutes, centrifuge the slurry and wash it 3 times to make it neutral, fully disperse the slurry in 60ml deionized water, transfer to 80ml poly Crystallized at 170°C for 6 hours in a vinyl fluoride reactor, then centrifuged and washed to obtain CoZnAl-LDH. The obtained CoZnAl-LDH slurry and 0.00225mol β-cyclodextrin were dispersed in 100ml deionized water at 80°C and stirred until all the water evaporated to obtain a CoZnAl-LDH / β-cyclodextrin complex.
[0026] The CoZnAl-LDH / β-cyclodextrin complex prepared above was placed in a tube atmosphere furnace under N 2 Under the atmosphere (80mL / min), the temperature was raised to 250...
Embodiment 3
[0029] 0.003mol (CH 3 COO) 2 Co 4H 2 O, 0.009mol (CH 3 COO) 2 Zn·2H 2 O, 0.003mol Al(NO 3 ) 3 9H 2 O was dissolved in 30ml deionized water as solution A. Another 0.024mol NaOH, 0.005mol CH 3 COONa was dissolved in 30ml deionized water as solution B. Slowly pour solution A and solution B into the colloid mill (4000rpm), fully stir and mix for 6 minutes, centrifuge the slurry and wash it 3 times to make it neutral, fully disperse the slurry in 60ml deionized water, transfer to 80ml poly Crystallized at 150°C for 10 hours in a vinyl fluoride reactor, then centrifuged and washed to obtain CoZnAl-LDH. The obtained CoZnAl-LDH slurry and 0.0015mol β-cyclodextrin were dispersed in 100ml deionized water at 80°C and stirred until all the water evaporated to obtain a CoZnAl-LDH / β-cyclodextrin complex.
[0030] The CoZnAl-LDH / β-cyclodextrin complex prepared above was placed in a tube atmosphere furnace under N 2 Under the atmosphere (100mL / min), the temperature was raised to 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com