Food additive comprising an amidase for detoxifying ochratoxin
A technology for food additives and ochratoxin, applied in food preparation, enzymes, enzymes, etc., can solve problems such as identification and display of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1O
[0337] Example 1 OTA activity screening
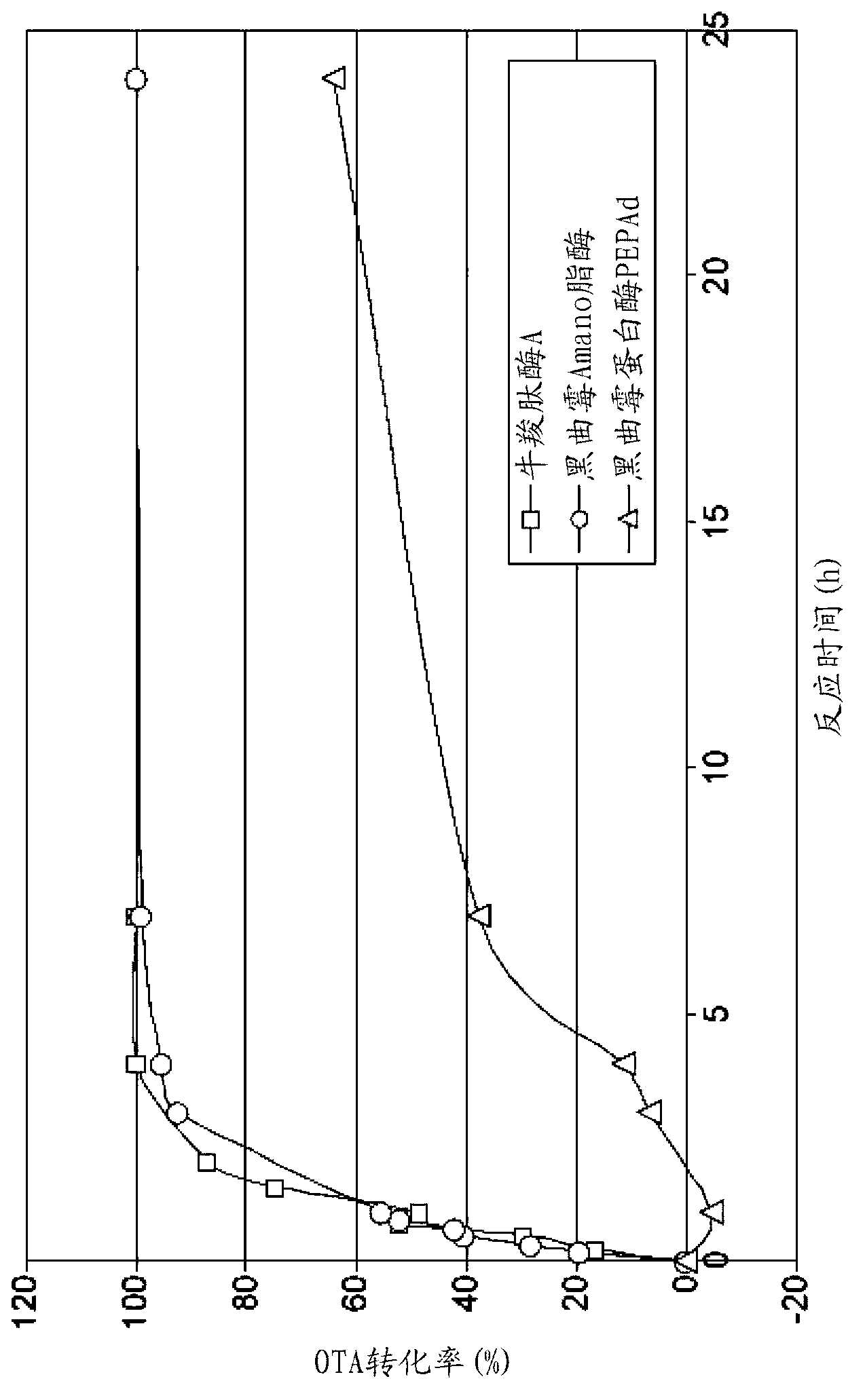
[0338] Thirty enzymes including various lipases, proteases and amidases were investigated for their ability to degrade ochratoxin A (Table 1). Only carboxypeptidase A and lipase from Aspergillus niger (Amano TM A) Can decompose OTA, as previously reported (Pitout, 1969; Stander et al., 2000). In addition, the aspartic protease pepAd2 (Danisco) from Aspergillus niger showed OTA conversion activity, but this activity was inefficient. In order to facilitate the comparison of these three enzymes, the protein concentration of PEPAd was determined and the same concentration (1 mg / ml) of carboxypeptidase A and Amano TM Enzyme solution of lipase A. Perform degradation kinetics ( figure 1 ). figure 1 Show Amano TM The OTA degradation kinetics of lipase A and carboxypeptidase A were similar. After 7 hours of reaction at 37°C, no OTA peak was visible, implying complete conversion of OTA. For PEPAd, complete conversion was not achieved...
Embodiment 2
[0342] Example 2 Purification of ochratoxin from Aspergillus niger fermentation broth or filtered fermentation broth (cell-free) A degradation activity .
[0343] First, ammonium sulfate is used for precipitation. Several concentrations of ammonium sulfate were applied to the crude A. niger protein extract. OTA degradation activity was determined in supernatants and centrifuged pellets. By increasing the ammonium sulfate concentration, the OTA degradation activity was transferred from the supernatant to the centrifuged pellet. At 60% ammonium sulfate, all degradative activity exists in the centrifuge pellet. This shows that enzymes from 40% to 60% ammonium sulphate produced precipitates are capable of hydrolyzing mycotoxins. At the same salt concentration (60%), slight lipase activity remained in the centrifuged pellet (data not shown).
[0344] Fractions from 40% to 60% ammonium sulfate were used as starting material for further ochratoxin degrading enzyme purificatio...
Embodiment 3
[0357] Example 3 Improved Purification of Ochratoxin A Degrading Enzyme
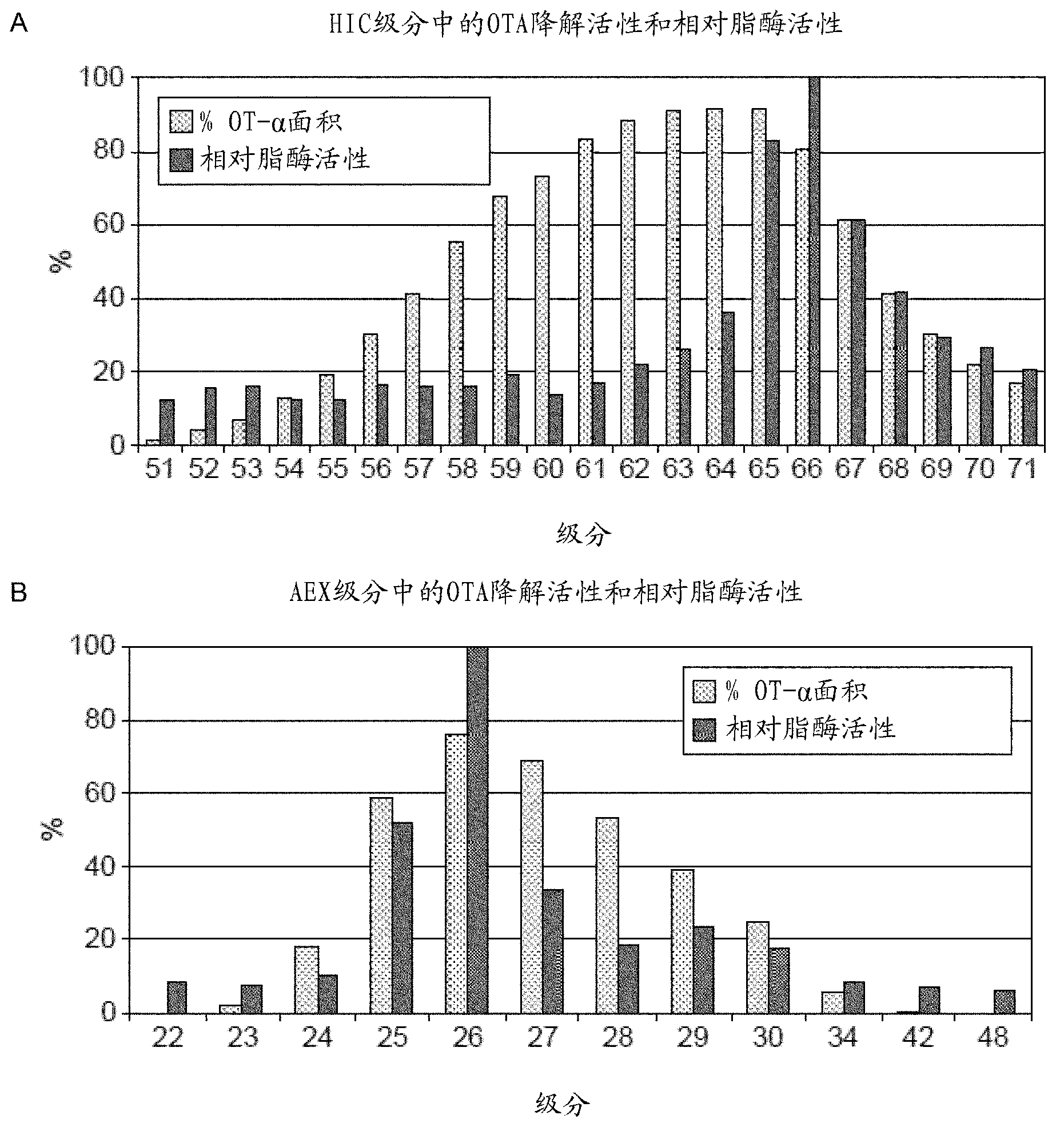
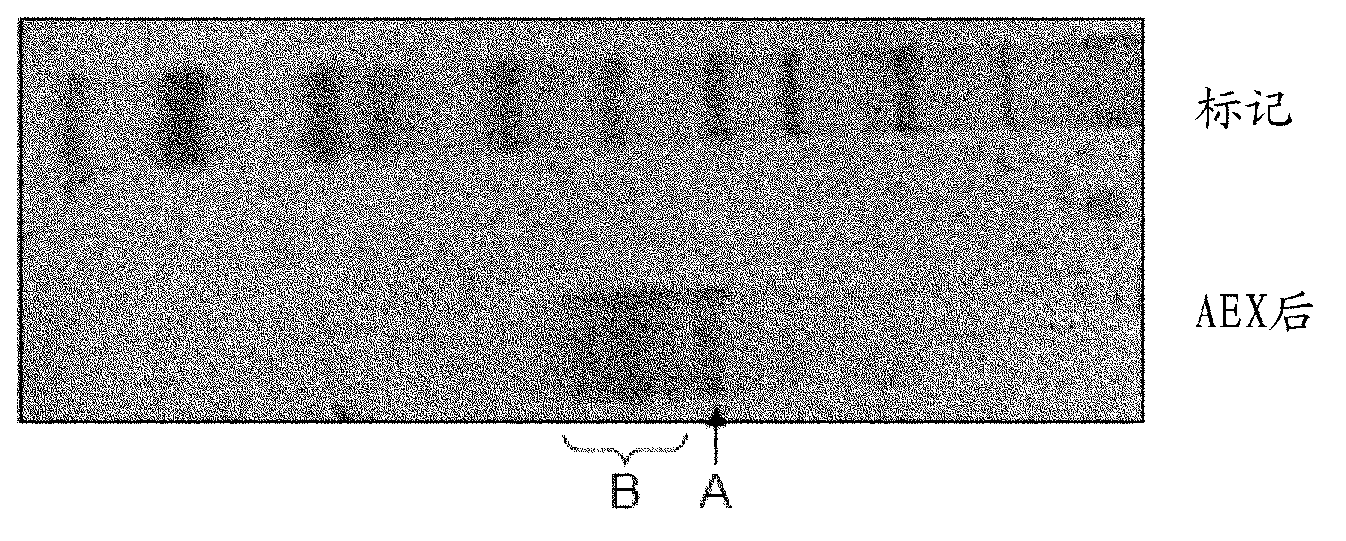
[0358] Further improvements in purification by HIC and AEX could not be achieved by using different buffers and different gradient programs. Purification using phenyl sepharose can be considered as an affinity step because the OTA molecule has phenyl groups, however, impurities are highly hydrophobic and bind tightly to the phenyl sepharose medium and elute very late ( Figure 4 A). When using octyl sepharose and butyl sepharose, the OTA degradation active fraction was present in the unadsorbed flow-through fraction. This suggests that it has low hydrophobicity and that its tight association with phenyl sepharose is due to the affinity properties ( Figure 4 A), while the impurity is highly hydrophobic, it binds tightly and elutes later ( Figure 4 B), as in the case of phenyl-agarose.
[0359] The procedure was further refined using ammonium sulfate fractionation (AS), HIC / affinity chromatography ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com