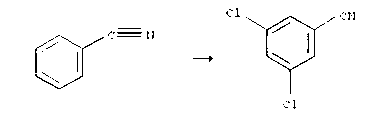Method for synthesizing 3,5-dichlorobenzoic acid
A technology of dichlorobenzoic acid and dichlorobenzoate is applied in the preparation of carboxylate, organic chemistry, nitrile preparation, etc., and can solve the problems of harsh production environment, long reaction time, and high cost of raw materials, and improve process safety. performance, low production cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
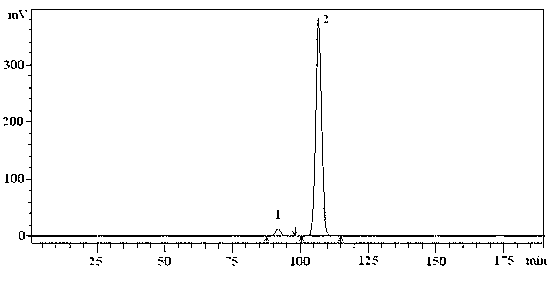
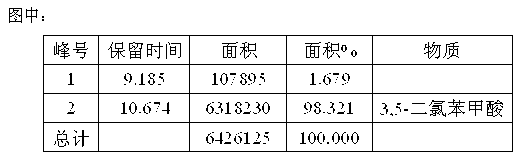
Image
Examples
Embodiment 1
[0027] Add 300g of chloroform and 100g of ethanol to 100.0g of benzonitrile (content 99%), and then add 1209.4g of sodium hypochlorite (content 13%), use 37% hydrochloric acid to keep the pH of the system at 3.0-4.0, at 55-60 At ℃, react until the content of 3,5-dichlorobenzonitrile is 99.5%, then add 30% sodium hydroxide under normal pressure to keep the pH value of the reaction system at 12-13, and keep the reaction at 80-90℃ After 2.5 hours, acidify the system with 37% hydrochloric acid until the pH value of the system is 1.0, react at 50-60°C for 1.0h, filter again, and dry at 90-110°C to obtain 182.5g of 3, with a content of 99.0%. 5-Dichlorobenzoic acid.
Embodiment 2
[0029] Add 1500g chloroform and 500g ethanol to 100.0g benzonitrile (content 99%) respectively, then add 1209.4g sodium hypochlorite (content 13%), use 98% sulfuric acid to keep the pH value of the system at 5.0-6.0, at 60-70 At ℃, react until the content of 3,5-dichlorobenzonitrile is 99.4%, and then add 20% sodium hydroxide under normal pressure to keep the pH value of the reaction system at 11-12, and keep the reaction at 80-90℃ After 3.0 hours, acidify the system with 37% hydrochloric acid until the pH value of the system is 0, react at 50-60°C for 0.5h, filter again, and dry at 90-110°C to obtain 182.5g of 3, with a content of 99.0%. 5-Dichlorobenzoic acid.
Embodiment 3
[0031] Add 75g of chloroform and 25g of ethanol to 100.0g of benzonitrile (content 99%), and then add 1209.4g of sodium hypochlorite (content 13%), use 25% hydrochloric acid to keep the pH value of the system at 0-1.0, at 55-70 At ℃, react until the content of 3,5-dichlorobenzonitrile is 99.5%, then add 10% sodium hydroxide under normal pressure to keep the pH value of the reaction system at 13-14, and keep the reaction at 80-90℃ After 2.7 hours, acidify the system with 37% hydrochloric acid until the pH value of the system is 1.5, react at 50-60°C for 0.6h, filter again, and dry at 90-110°C to obtain 182.3g of 3, with a content of 99.0%. 5-Dichlorobenzoic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com