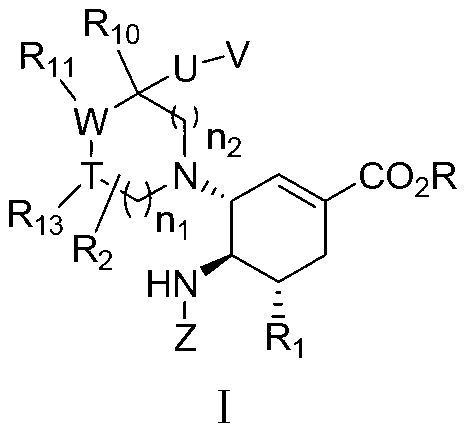
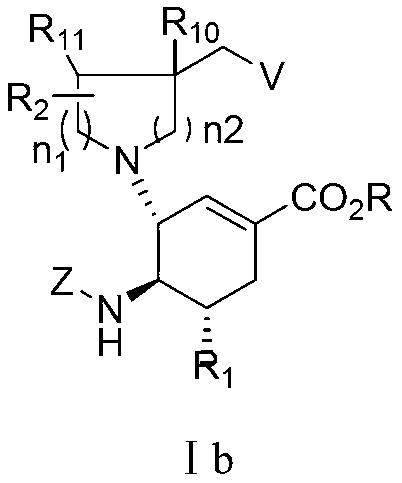
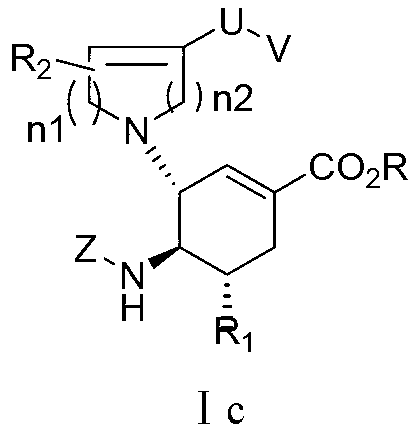
Cyclohexene derivative or its pharmaceutically acceptable salt and application thereof
A technology of cyclohexene and derivatives, which is applied in the field of cyclohexene derivatives and can solve problems such as limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1 Compound 3-1: (3R,4R,5S)-4-acetamido-5-amino-3-(3(S)-ethoxypiperidine)-1-cyclohexene-1-carboxy acid preparation.
[0124] 1. Preparation of intermediate 1.
[0125] Using shikimic acid as the starting material, the intermediate 1 ((3R,4R,5S)-3-acetoxy-4-acetamido-5-tert-butoxyamido-1-cyclohexyl was prepared after conversion Alkene-1-carboxylate ethyl ester), the reaction process is as follows:
[0126]
[0127] 2. Preparation of (3R,4R,5S)-4-acetamido-5-tert-butylcarboxamido-3-(3(S)-ethoxypiperidine)-1-cyclohexene-1-carboxylic acid .
[0128] Dissolve intermediate 1 (1 mmol) and tetrakistriphenylphosphine palladium (58 mg, 0.05 mmol) in 5 mL of DMF under nitrogen protection, add DIPEA (0.6 mL, 3 mmol), stir for 5 minutes, and then add 3S-ethoxypiperidine Pyridine in DMF. Stir at room temperature for 5 minutes, then heat the reaction at 80°C until the end (about 2h). After cooling to room temperature, 50 mL of ethyl acetate and 10 mL of saturated brine...
Embodiment 2
[0132] Example 2 Compound 5-1: Preparation of (3R,4R,5S)-4-acetamido-5-piperyl-3-S-ethoxypiperidine-1-cyclohexene-1-carboxylic acid.
[0133] Take the compound 3-1 prepared in Example 1, dissolve it in DMF solution, add 1H-pyrazole-1-carboxamidine and triethylamine, and stir at room temperature for 8 hours. After the solvent was distilled off under reduced pressure, it was redissolved in ethyl acetate, and the pH was adjusted to 2-3 with 1M HCl. The precipitate was filtered to obtain compound 5-1.
[0134] The characterization data of compound 5-1 is: ESI-MS m / z: 368(M+1). 1 H NMR (400MHz,MeOD)δ6.96(s,1H),4.51–4.32(m,2H),4.02–3.89(m,1H),3.85(br,1H),3.65-3.45(m,4H), 3.35(s,1H),3.00(dd,J=17.9,4.9Hz,1H),2.46(dd,J=17.5,10.5Hz,1H),2.26(d,J=38.9Hz,1H),2.08(d ,J=17.5Hz,3H),1.82(d,J=14.9Hz,1H),1.33-1.40(m,3H),1.20(t,J=6.9Hz,3H), 13 CNMR (126MHz, MeOD) δ175.56, 167.35, 158.85, 137.85, 127.35, 68.56, 65.53, 55.82, 54.75, 51.79, 50.56, 43.77, 31.45, 22.70, 18.70, 17.28, 15.60.
Embodiment 3
[0135] Example 3 Compound 3-2: (3R,4R,5S)-4-acetamido-5-amino-3-(3(S)-acetamidopiperidine)-1-cyclohexene-1-carboxy acid preparation.
[0136] 1. Preparation of Intermediate 1: Same as Example 1.
[0137] 2. (3R,4R,5S)-4-acetamido-5-tert-butylformylamino-3-(3(S)-acetamidopiperidine)-1-cyclohexene-1-carboxylic acid preparation.
[0138] Dissolve intermediate 1 and tetrakistriphenylphosphine palladium in DMF under nitrogen protection, add DIPEA, stir for 5 minutes, then add 3S-acetamidopiperidine in DMF, and heat the reaction until the end. Cool to room temperature, add ethyl acetate and saturated brine for extraction, and dry the organic phase over anhydrous sodium sulfate. Ethyl acetate was distilled off under reduced pressure, and compound 2-2 was obtained after separation by column chromatography.
[0139] 3. Preparation of compound 3-1.
[0140] Compound 2-2 was dissolved in tetrahydrofuran, and the rest of the operations were the same as in Example 1 to obtain compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com