Calcium-sensing receptor-active compounds
A kind of technology of compound and stereoisomer, applied in the field of preparation of medicament
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
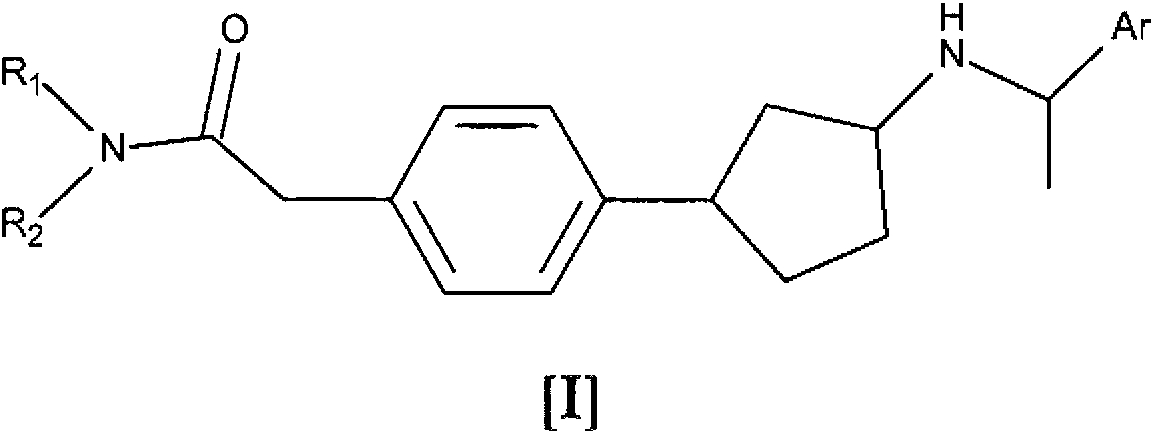
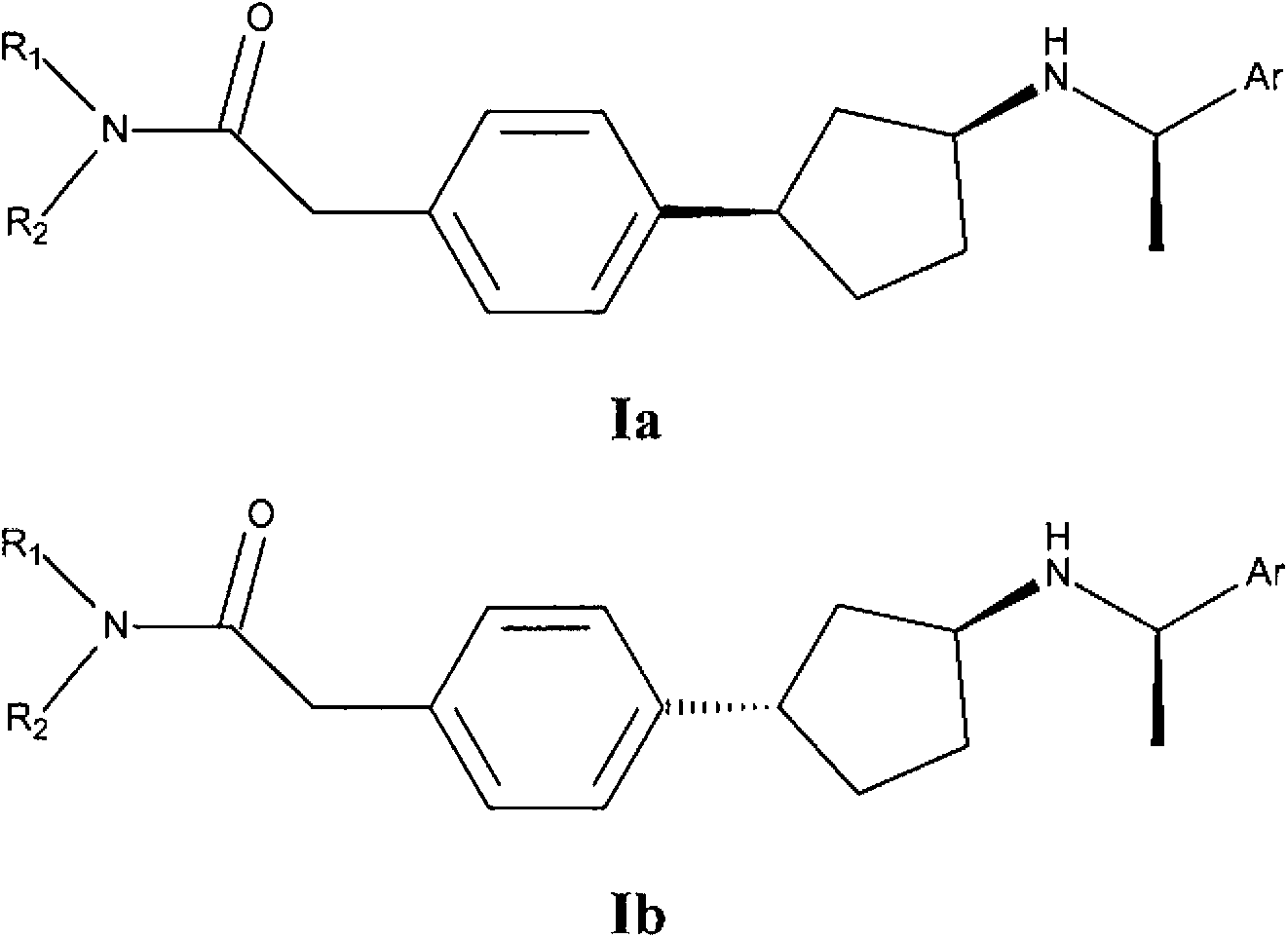
[0061] In one embodiment of the invention, compound I represents Ia or Ib
[0062]
[0063] In one embodiment of the invention Ar represents phenyl optionally substituted by one or more identical or different substituents independently selected from chlorine, fluorine or C 1-3 alkoxy.
[0064] In one embodiment of the invention Ar represents phenyl substituted by one or two identical or different substituents independently selected from chlorine, fluorine or methoxy.
[0065] In one embodiment of the invention Ar represents 4-fluoro-3-methoxyphenyl, 3-chlorophenyl or 3-ethoxyphenyl.
[0066] In one embodiment of the invention, Ar represents C comprising 1-3 heteroatoms selected from O 2-5 Heterocycloalkylphenyl.
[0067] In one embodiment of the invention, R 1 means C 1-4 Alkyl, C 2-4 Alkenyl, C 2-4 Alkynyl, hydroxyl C 2-4 Alkyl, amino C 2-4 Alkyl, hydroxyl C 2-4 Alkylamino C 2-4 Alkyl, C 1-2 Alkylsulfonylamino C 2-4 Alkyl, aminosulfonyl C 1-4 Alkyl, aminocarb...
Embodiment 1
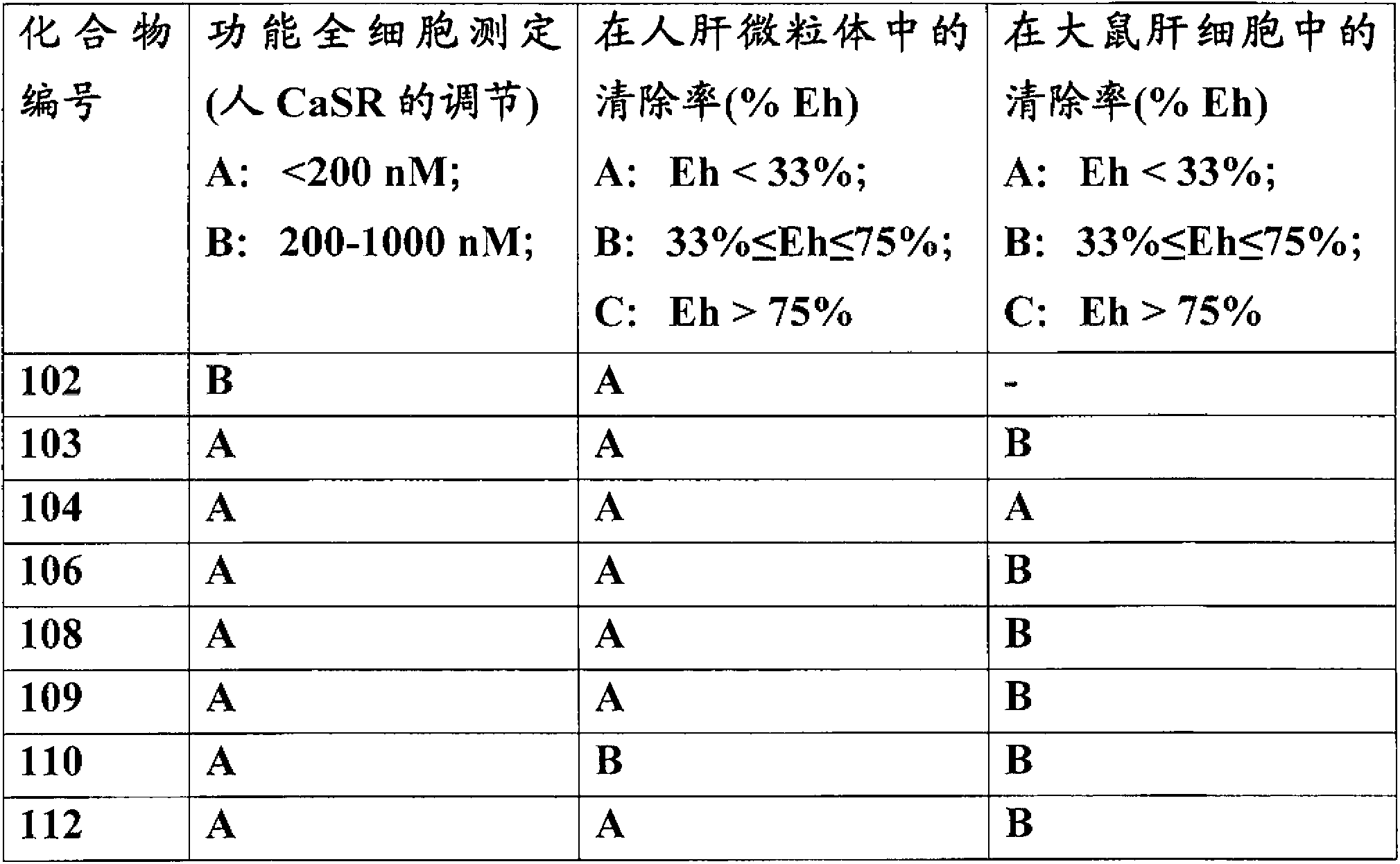
[0279] Example 1: 4-[2-[4-[(1R,3S)-3-[[(1R)-1-(4-fluoro-3-methoxy-phenyl)ethyl]-amino]- ring Pentyl]phenyl]acetyl]piperazin-2-one; formic acid (compound 101)
[0280] Following general procedure B, intermediate 3 was used as the acid and piperazin-2-one as the amine.
[0281] 1 H NMR (300MHz, DMSO) δ8.25(s, 1H), 8.09 / 8.01(s, 1H, rotamer), 7.26(dd, J=8.5, 1.8Hz, 1H), 7.20-7.07(m, 5H), 6.96(ddd, J=8.2, 4.4, 1.9Hz, 1H), 4.08-3.89(m, 3H), 3.84(s, 3H), 3.73-3.55(m, 4H), 3.18-2.97(m, 3H), 2.96-2.80(m, 1H), 2.20-2.05(m, 1H), 1.97-1.60(m, 4H), 1.53-1.38(m, 1H), 1.35(d, J=6.6Hz, 3H) .
Embodiment 2
[0282] Example 2: 2-[4-[(1R,3S)-3-[[(1R)-1-(4-fluoro-3-methoxy-phenyl)ethyl]amino]-cyclopentyl] Phenyl]-N-[2-(methylsulfonylamino)ethyl]acetamide; Formic acid (compound 102)
[0283] Following general procedure B, intermediate 3 was used as the acid and N-(2-aminoethyl)methanesulfonamide hydrochloride as the amine.
[0284] 1 H NMR (600MHz, DMSO) δ8.31(s, 1H), 8.13(t, J=5.8Hz, 1H), 7.30(dd, J=8.4, 1.8Hz, 1H), 7.20-7.12(m, 5H) , 7.09(t, J=5.4Hz, 1H), 6.99(ddd, J=8.2, 4.2, 2.0Hz, 1H), 4.03(q, J=6.6Hz, 1H), 3.84(s, 3H), 3.36( s, 2H), 3.15(dd, J=12.7, 6.4Hz, 2H), 3.12-3.06(m, 1H), 2.98(dd, J=12.1, 6.3Hz, 2H), 2.92-2.84(m, 1H) , 2.87(s, 3H), 2.17-2.10(m, 1H), 1.94-1.84(m, 2H), 1.81-1.73(m, 1H), 1.72-1.64(m, 1H), 1.50(td, J= 11.9, 9.4 Hz, 1H), 1.39 (d, J = 6.7 Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com