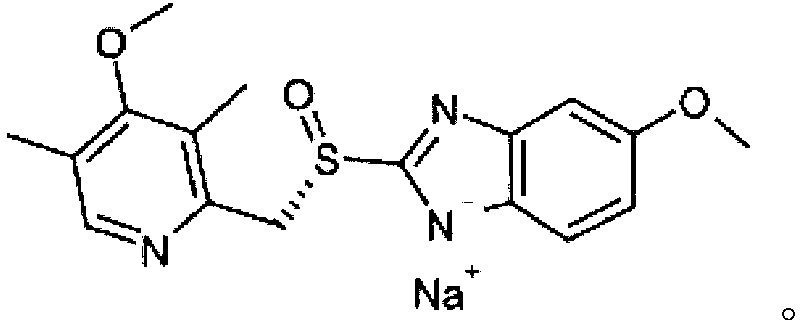
Esomeprazole sodium or omeprazole sodium content determination method
A technology of esomeprazole sodium and omeprazole sodium, which is applied in the field of content determination of esomeprazole sodium or omeprazole sodium, and can solve the problems of difficult judgment of the end point, difficult cleaning, small jump, etc. , to achieve the effect of avoiding cleaning work, easy operation and large jump value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 The detection method of esomeprazole sodium of the present invention
[0023] (1) Blank test: Take 50ml of ethanol-water (20%:80%), and titrate with hydrochloric acid titrant (0.1mol / L) according to the potentiometric titration method (Chinese Pharmacopoeia 2010 Edition Two Appendix ⅦA).
[0024] (2) Determination of the test product: Take about 0.3g of the test product, accurately weigh it, add 50ml of ethanol-water (20%:80%) to dissolve it, and follow the potentiometric titration method (Chinese Pharmacopoeia 2010 Edition Two Appendix ⅦA) , Titrate with hydrochloric acid titrant (0.1mol / L).
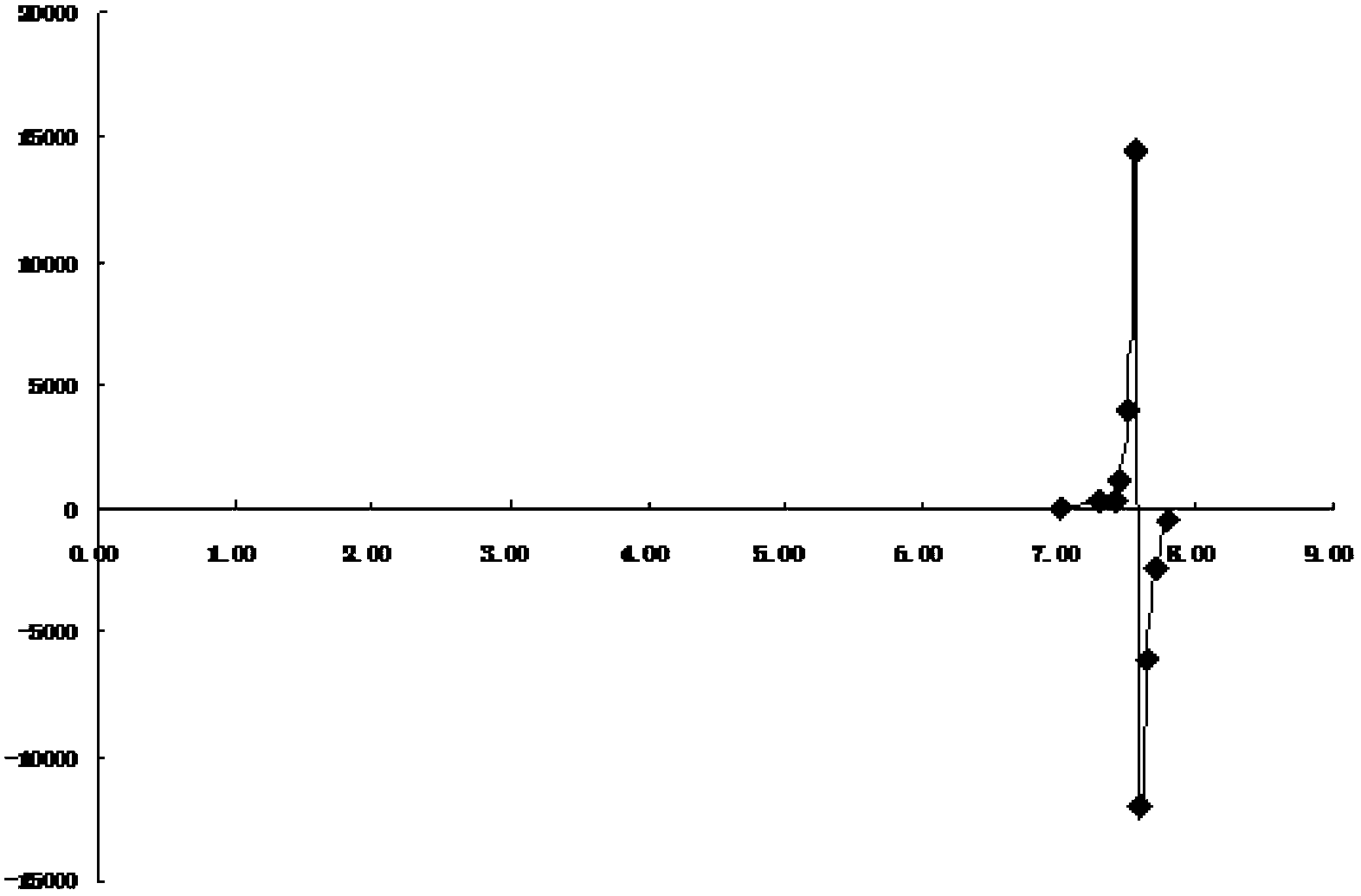
[0025] (3) The titration data is processed by the second-order reciprocal method, and the endpoint volume is calculated. The titration data and content determination results are shown in Table 1, and the second-order reciprocal graph is shown in Table 1. figure 1 .
[0026] Table 1 Ethanol-water (20%: 80%) titration data and content determination results
[0027]
[0028]
[00...
Embodiment 2
[0043] Example 2 Investigation of the detection method of the present invention
[0044] 1. Screening of ethanol-water ratio
[0045] 1.1 Blank test: Take 50ml of ethanol-water (20-100%:0-80%), and titrate with hydrochloric acid titrant (0.1mol / L) according to the potentiometric titration method (Chinese Pharmacopoeia 2010 Edition Two Appendix ⅦA).
[0046] 1.2 Determination of the test product: Take about 0.3g of the test product, add 50ml of ethanol-water (20-100%:0-80%) to dissolve it, and use the potentiometric titration method (Appendix ⅦA of the Second Part of Chinese Pharmacopoeia 2010 Edition). Titration with hydrochloric acid titrant (0.1mol / L).
[0047] 1.3 Test phenomena and results
[0048] 1.3.1 The potential at each point is stable, and no more precipitation and viscous substances are formed.
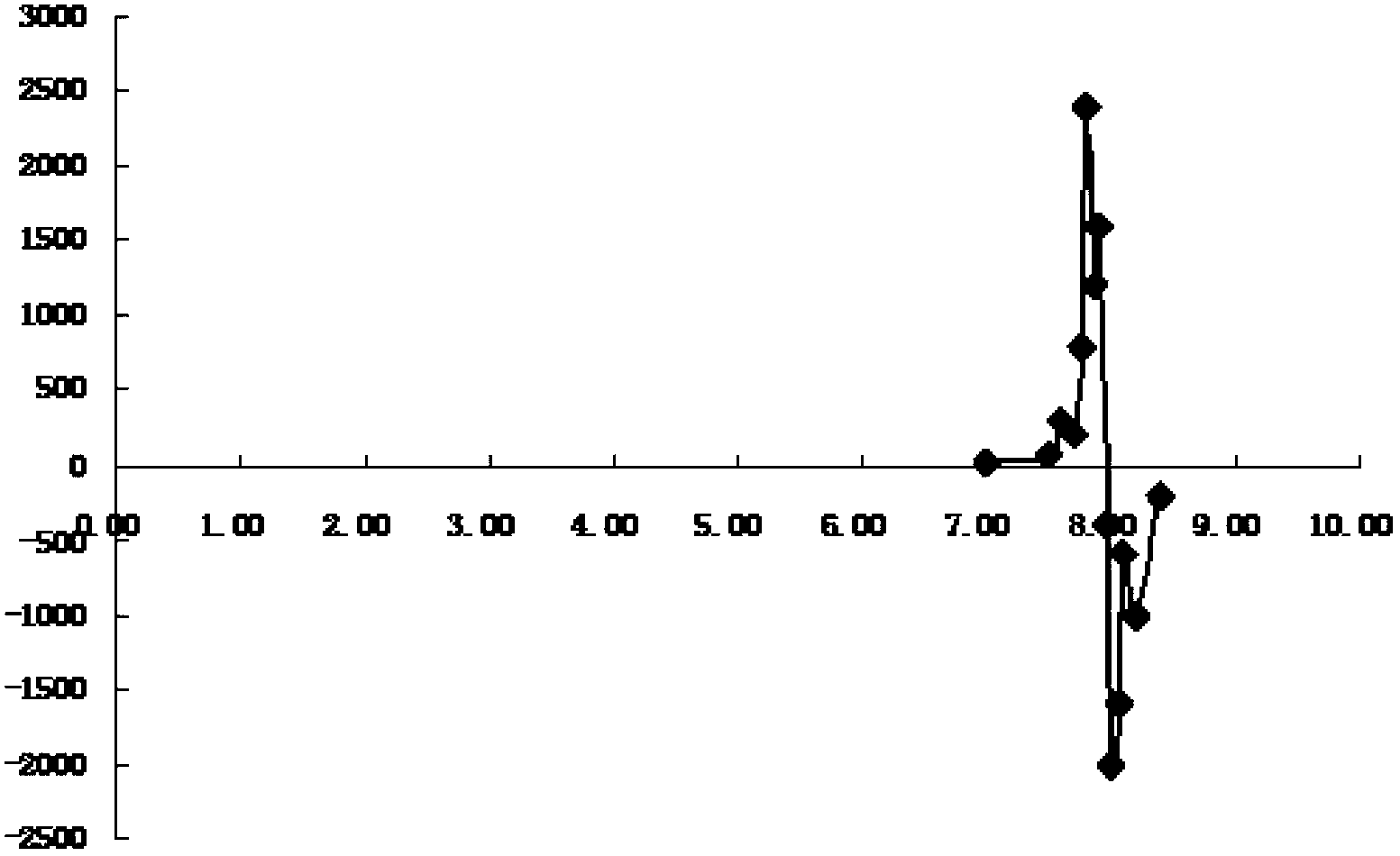
[0049] 1.3.2 The titration data and content determination results are shown in Table 3-7 (the titration data is processed by the second-order reciprocal method, and the endpoint vol...
Embodiment 3
[0088] Example 3 Methodological investigation
[0089] 1. Repeatability test:
[0090] 1.1 Blank test: Take 50ml of ethanol-water (20%:80%) and titrate with hydrochloric acid titrant (0.1mol / L) according to the potentiometric titration method (Chinese Pharmacopoeia 2010 Edition Two Appendix ⅦA).
[0091] 1.2 Determination of the test product: Take 6 test samples, each about 0.3g, add 50ml ethanol-water (20%:80%) to dissolve them, and follow the potentiometric titration method (Chinese Pharmacopoeia 2010 Edition Two Appendix ⅦA), Titrate with hydrochloric acid titrant (0.1mol / L).
[0092] 1.3 The titration data is processed by the second-order reciprocal method, and the endpoint volume is calculated. The titration data is shown in Table 13, and the content inspection result is shown in Table 14.
[0093] Table 13 Titration data of repeatability test using the present invention
[0094]
[0095]
[0096] Table 14 The content repeatability determination results of the present invention
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com