Preparation method for lithium tetrafluoroborate
A technology of lithium tetrafluoroborate and lithium carbonate, which is applied in the direction of borates, boron oxide compounds, and educts, can solve the problems of reducing the purity of lithium tetrafluoroborate, increasing production costs, and affecting the dehydration effect, achieving huge environmental benefits and social benefits, increase output rate, and improve product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
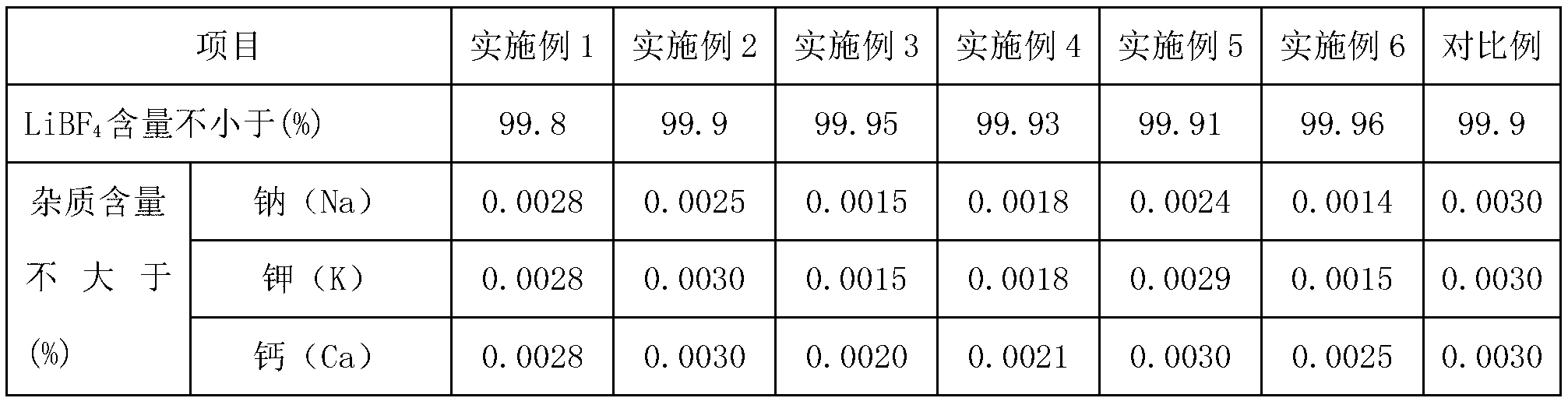
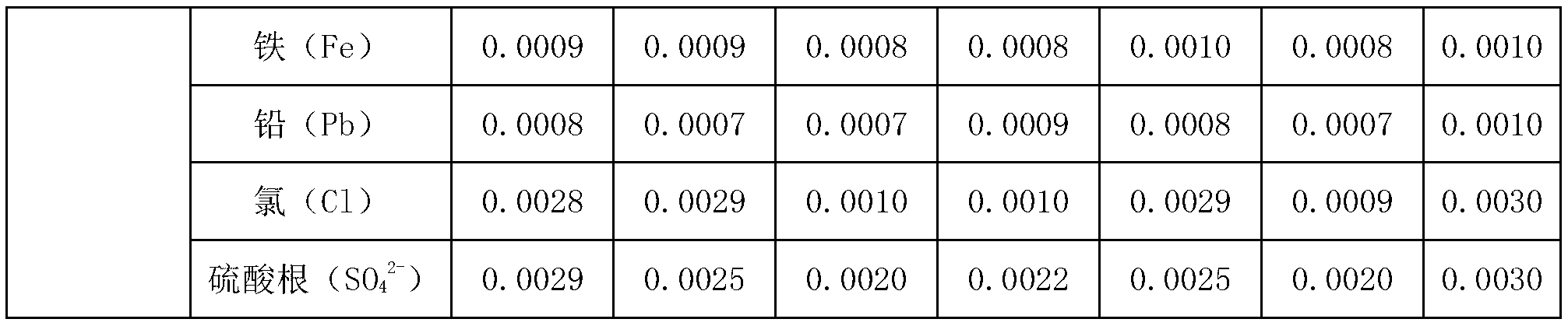
Examples
Embodiment 1
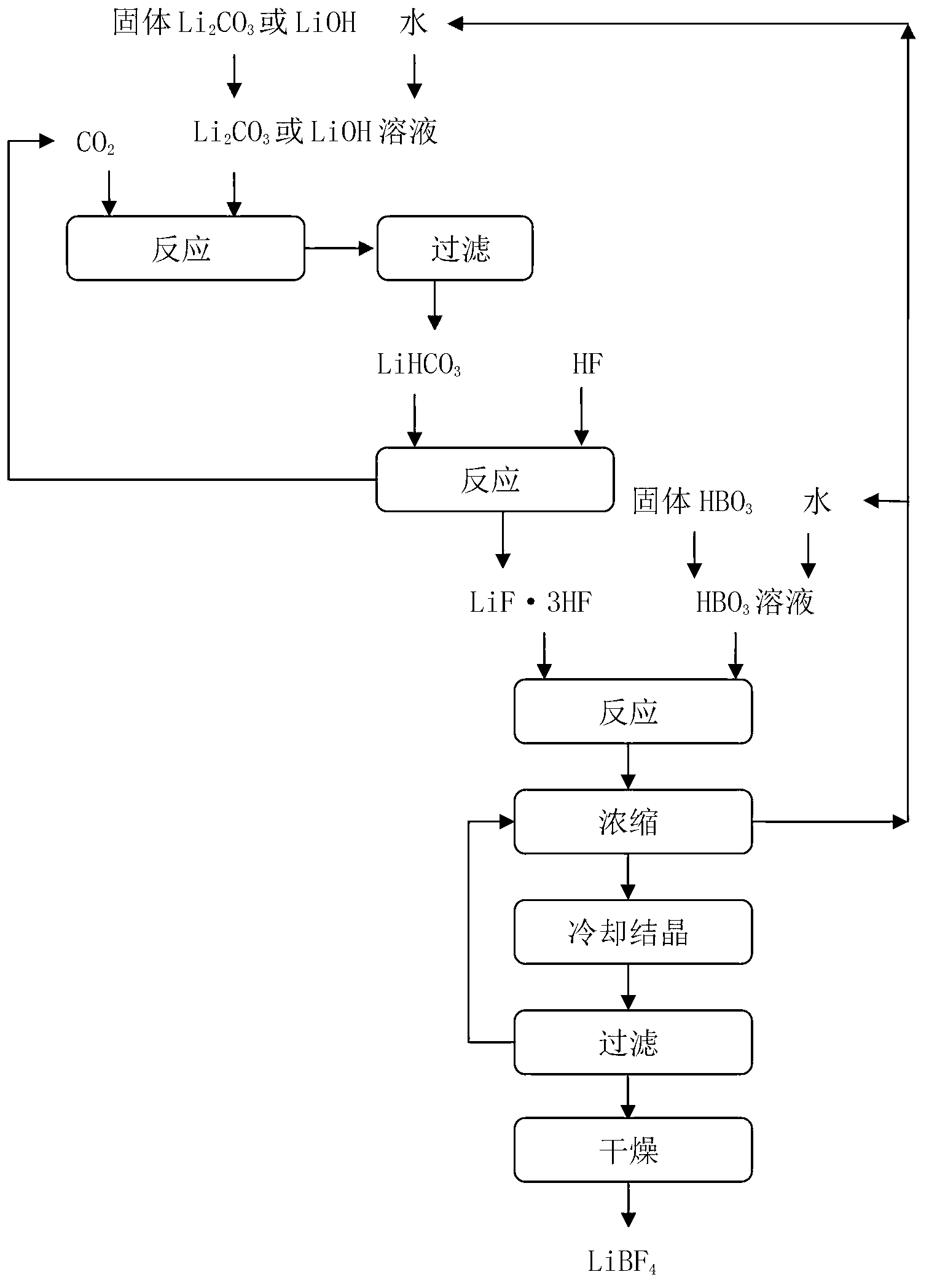
[0043] The preparation method of lithium tetrafluoroborate of the present embodiment, technological process is as follows figure 1 shown, including the following steps:
[0044] 1) Lithium carbonate is prepared into a slurry with a mass fraction of 5%, and CO is continuously fed under a pressure of 1MPa 2 , the reaction produces LiHCO 3 , the reaction solution was filtered to obtain LiHCO 3 solution; the accuracy of the filtration is: the removal rate of solid particles with a particle size of 1 μm or more is greater than 99.9%;
[0045] 2) The LiHCO obtained in step 1) 3 solution with anhydrous hydrofluoric acid in LiHCO 3 Mix with anhydrous hydrofluoric acid at a molar ratio of 1:4, and react at room temperature for 2.5 hours to obtain LiF 3HF solution;
[0046] 3) Mix the LiF·3HF solution obtained in step 2) with the boric acid solution with a mass fraction of 5% at a molar ratio of LiF·3HF to boric acid of 1:1, and react to obtain a lithium tetrafluoroborate solution;...
Embodiment 2
[0049] The preparation method of lithium tetrafluoroborate of the present embodiment, technological process is as follows figure 1 shown, including the following steps:
[0050] 1) Lithium carbonate is prepared into a slurry with a mass fraction of 10%, and CO is continuously fed under a pressure of 5MPa 2 , the reaction produces LiHCO 3 , the reaction solution was filtered to obtain LiHCO 3 solution; the accuracy of the filtration is: the removal rate of solid particles with a particle size of 1 μm or more is greater than 99.9%;
[0051] 2) The LiHCO obtained in step 1) 3 solution with anhydrous hydrofluoric acid in LiHCO 3 Mix with anhydrous hydrofluoric acid at a molar ratio of 1:5, and react at room temperature for 5 hours to obtain LiF 3HF solution;
[0052] 3) Mix the LiF·3HF solution obtained in step 2) with the boric acid solution with a mass fraction of 20% at a molar ratio of LiF·3HF to boric acid of 1.3:1, and react to obtain a lithium tetrafluoroborate solutio...
Embodiment 3
[0055] The preparation method of lithium tetrafluoroborate of the present embodiment, technological process is as follows figure 1 shown, including the following steps:
[0056] 1) Lithium carbonate is prepared into a slurry with a mass fraction of 0.5%, and CO is continuously fed under a pressure of 2MPa 2 , the reaction produces LiHCO 3 , the reaction solution was filtered to obtain LiHCO 3 solution; the accuracy of the filtration is: the removal rate of solid particles with a particle size of 1 μm or more is greater than 99.9%;
[0057] 2) The LiHCO obtained in step 1) 3 solution with anhydrous hydrofluoric acid in LiHCO 3 Mix with anhydrous hydrofluoric acid at a molar ratio of 1:4.5, and react at room temperature for 0.5h to obtain LiF 3HF solution;
[0058] 3) Mix the LiF·3HF solution obtained in step 2) with the boric acid solution with a mass fraction of 30% at a molar ratio of LiF·3HF to boric acid of 1.5:1, and react to obtain a lithium tetrafluoroborate solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com