Method for preparing copper chloride dihydrate by alkaline type copper chloride
A technology of copper chloride dihydrate and copper chloride, applied in the direction of copper chloride, copper halide, etc., can solve the problems of unsuitable for large-scale production, cumbersome reaction steps, high production cost, etc., achieve improved production efficiency, simple preparation method, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A method for preparing copper chloride dihydrate using basic copper chloride, comprising:
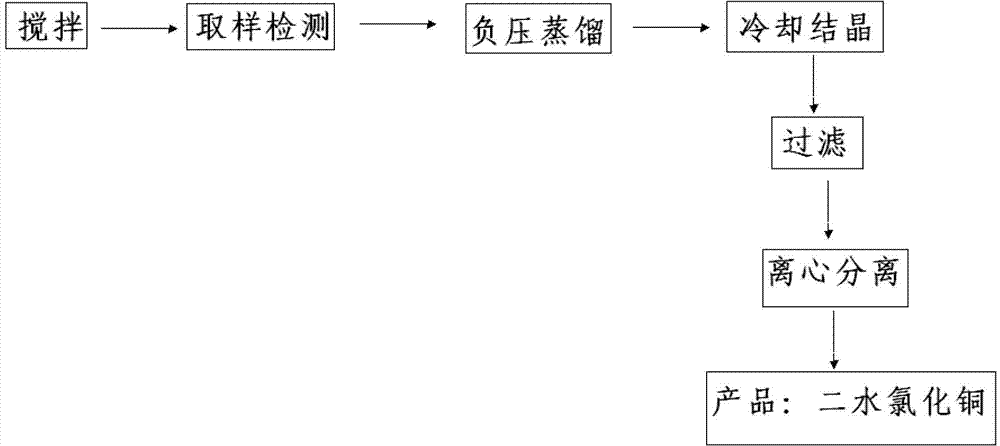
[0030] (1) Add 2m 3 Concentration is 27% hydrochloric acid, start to stir, then add 1.5t concentration to be 55% basic copper chloride in hydrochloric acid. After 20-40 minutes of feeding, take a sample to detect free hydrogen chloride, and the concentration of free hydrogen chloride needs to be controlled at 0.8%-8%. At the same time, the above-mentioned additions need to be completely dissolved without sediment.
[0031] (2) After the feeding is completed, the test is qualified, and the vacuum distillation is started for 8-10 hours. The selected vacuum degree is -0.05Mpa. When the material is steamed to a thick slurry and the surface has large bubbles, the evaporation is over, and the steam valve of the reactor is closed. Cooling water, lowering the temperature to 40°C, putting the feed liquid into a filter barrel, filtering the mother liquor, and centrifuging for 40 minutes ...
Embodiment 2
[0033] A method for preparing copper chloride dihydrate using basic copper chloride, comprising:
[0034] (1) Add 1.4m 3 Concentration of 32% hydrochloric acid, start stirring, vacuumize to make the vacuum degree -0.05Mpa, then add 1.4t of basic copper chloride with a concentration of 58% to the hydrochloric acid. After 20-40 minutes of feeding, take a sample to detect free hydrogen chloride, and the concentration of free hydrogen chloride needs to be controlled at 0.8%-8%. At the same time, the above-mentioned additions need to be completely dissolved without sediment.
[0035] (2) After the feeding is completed, the test is qualified, and the vacuum distillation is started for 8-10 hours. The selected vacuum degree is -0.12Mpa. When the material is steamed to a thick slurry and the surface has large bubbles, the evaporation is over, and the steam valve of the reactor is closed. Cooling water, lowering the temperature to 45°C, putting the feed liquid into a filter barrel, f...
Embodiment 3
[0037] A method for preparing copper chloride dihydrate using basic copper chloride, comprising:
[0038] (1) Add 1.9m 3 Concentration of 28% hydrochloric acid, start stirring (rotational speed 700-1000r / min), vacuumize to make the vacuum degree -0.10Mpa, then add 1.6t basic copper chloride with a concentration of 54% to the hydrochloric acid. After 20-40 minutes of feeding, take a sample to detect free hydrogen chloride, and the concentration of free hydrogen chloride needs to be controlled at 0.8%-8%. At the same time, the above-mentioned additions need to be completely dissolved without sediment.
[0039] (2) After the feeding is completed, the test is qualified, and the vacuum distillation is started for 8-10 hours. The selected vacuum degree is -0.12Mpa. When the material is steamed to a thick slurry and the surface has large bubbles, the evaporation is over, and the steam valve of the reactor is closed. Cool water, lower the temperature to 30°C, put the feed liquid int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com