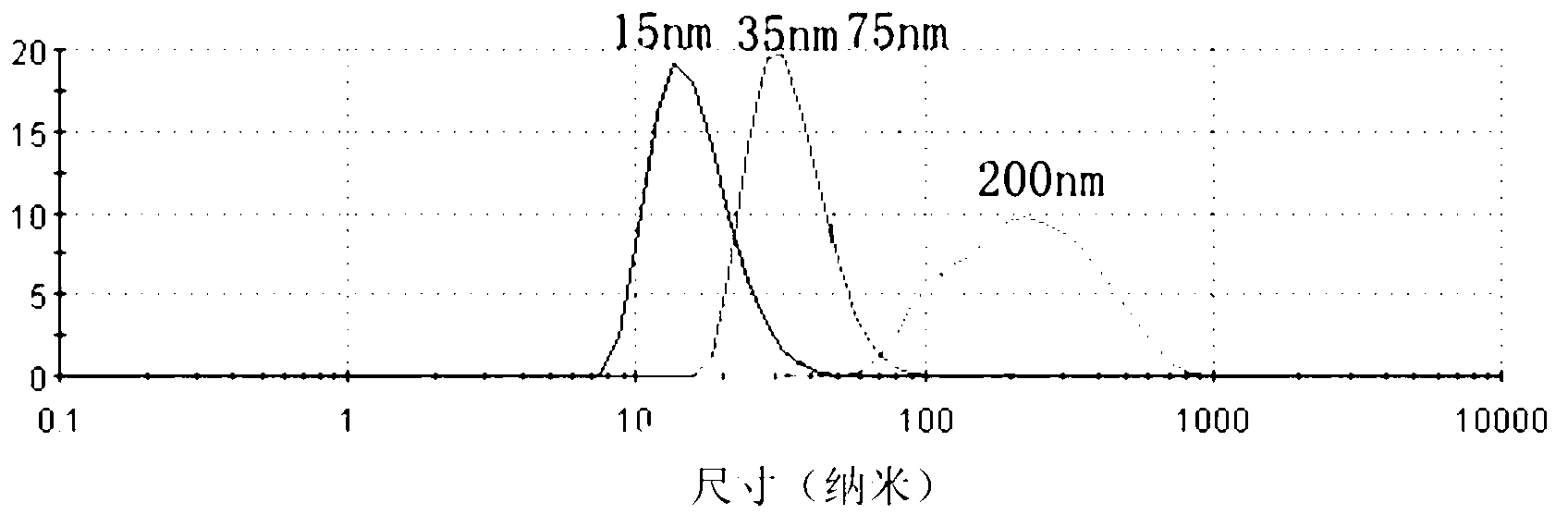
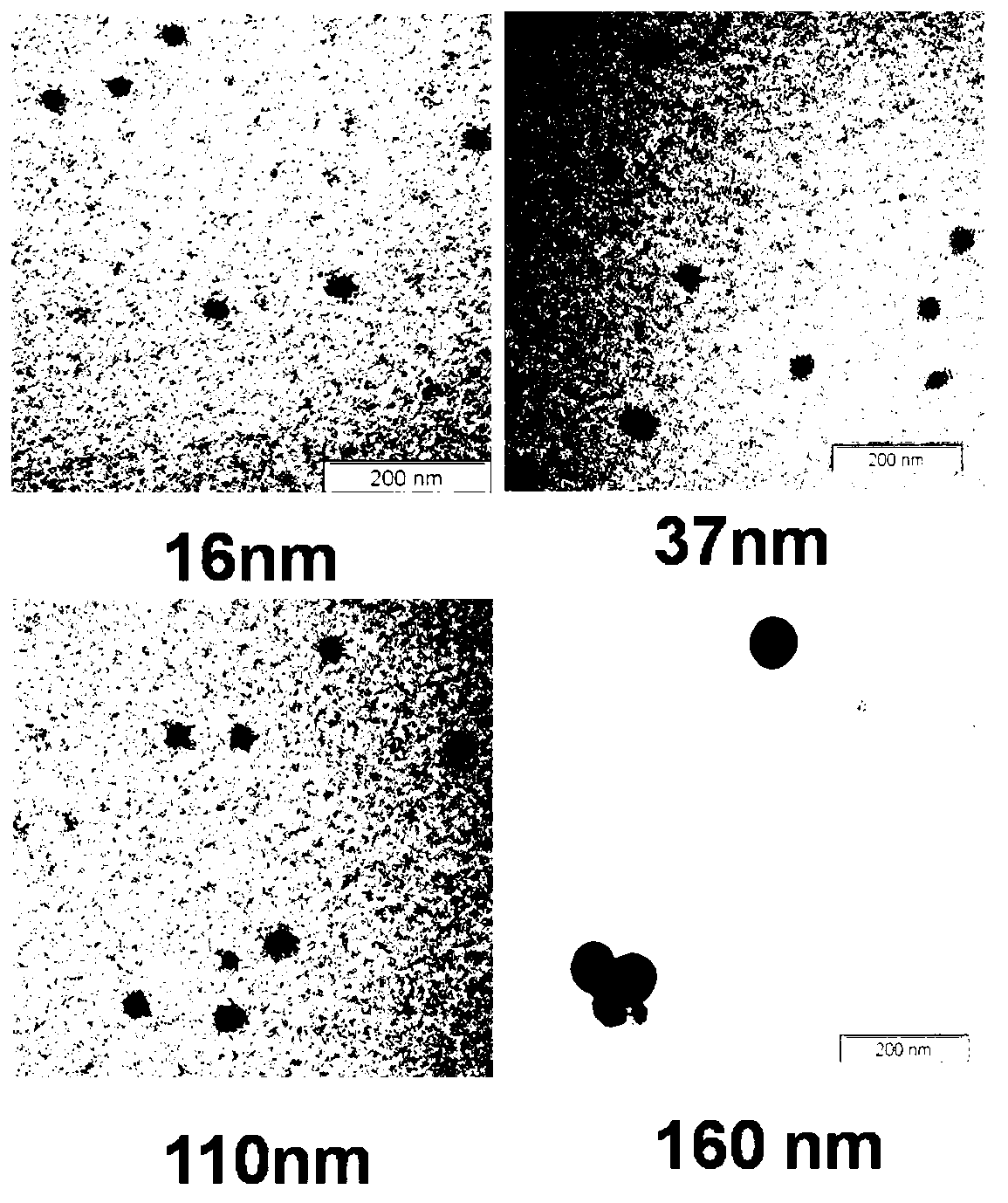
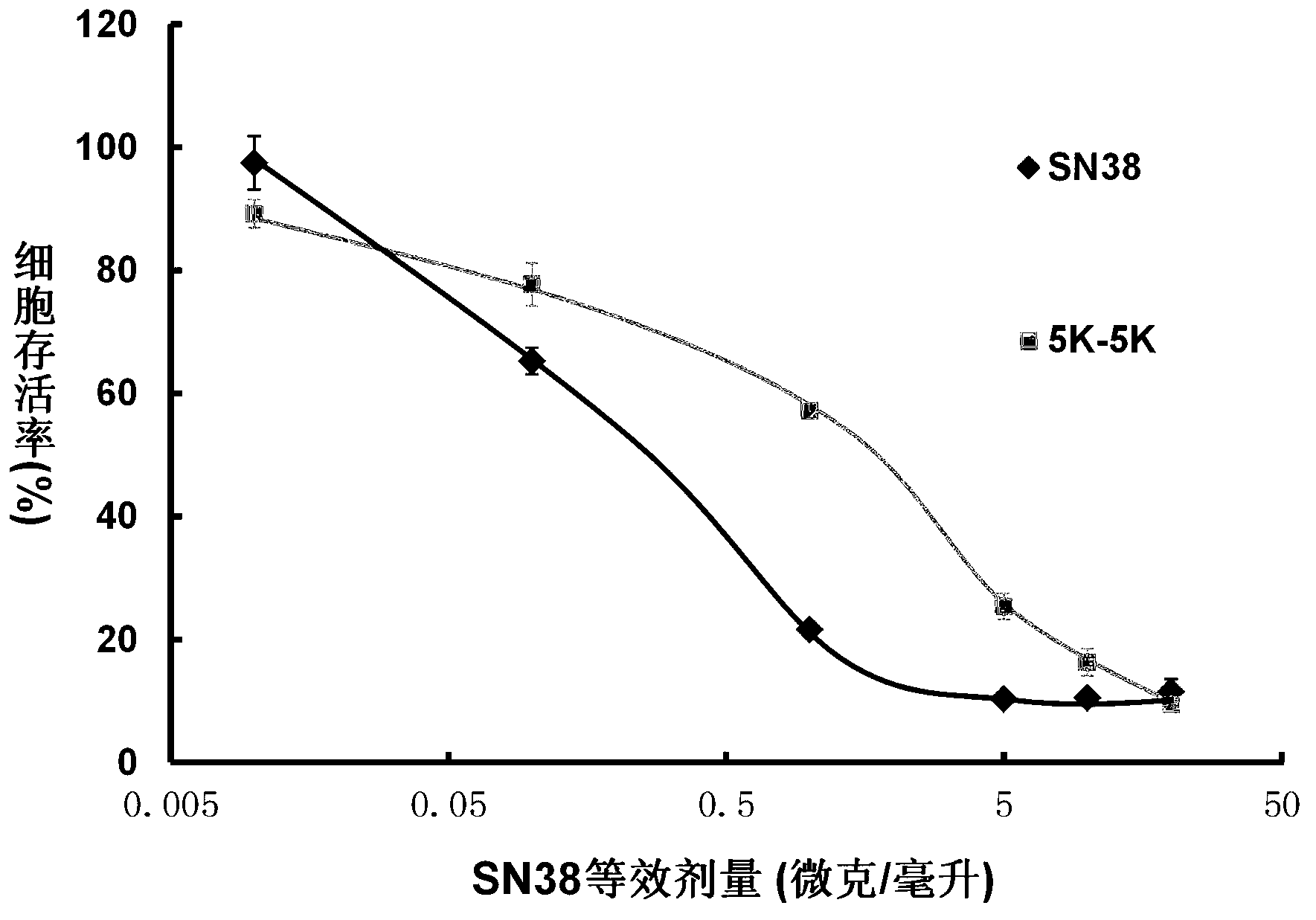
7-ethyl-10-hydroxycamptothecin amphiphilic polymer prodrug as well as preparation method and nano-particles thereof
A technology of amphiphilic polymers and hydroxycamptothecin, which is applied in the field of nanoparticles, can solve the problems of cumbersome preparation methods and unclear drug structures, and achieve the effects of simple preparation methods, less use of carriers, and reduced side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Polyethylene glycol (Mn=5000) polymer prodrug (PEG 5000 -P(HEMASN38) 5000 )Synthesis
[0055] Synthesis of HEMASN38
[0056] Mono[2-[(2-methyl-acryloyl)oxy]ethyl] succinate (Mn=230, 4.6g) and 7-ethyl-10-hydroxycamptothecin (Mn=392, 3.92g ) were mixed in dichloromethane, N,N'-dicyclohexylcarbodiimide (Mn=206, 4.1g) and triethylamine were added, and stirred at room temperature for 24 hours. Then washed with acid water, dried, concentrated and purified by column, the 1H-NMR (400M, CDCl 3 ) for: 8.254(d), 7.829(s), 7.68(s), 7.53(d), 6.12(s), 5.75(d), 5.56(s), 5.27(d), 5.26(s), 4.39( m), 3.13(q), 2.97(t), 2.81(t), 1.89(m), 1.38(t), 1.02(t). It was shown that the compound HEMASN38 (4.8 g, yield 80%) of the structure shown above was obtained.
[0057] Add polyethylene glycol bromoisobutyrate (Mn=5000, 0.5g) and HEMASN38 (Mn=604, 0.5g) into 5mL of anisole, then add CuBr (Mn=141, 2.8mg) and bipyridine (Mn=156, 6.3mg) as a catalyst, heated to 60°C, and reacted fo...
Embodiment 2
[0063] Example 2 The polymer prodrug (PEG5000 -P(HEMASN38) 5000 )Synthesis
[0064] (1) Synthesis of SN38 tert-butyl carbonate (Boc-SN38)
[0065] Mix di-tert-butyl dicarbonate (Mn=186, 2g) and SN38 (392, 2g) in dichloromethane (150mL), add 10mL of pyridine as a catalyst, stir for 24 hours, wash with dilute hydrochloric acid, anhydrous sodium sulfate After drying, 3g product was obtained, and the product was characterized by NMR ( Figure 7 ), proved to be the product Boc-SN38, and the yield was close to 100%.
[0066] Add Boc-SN38 (Mn=493, 0.5g) and mono[2-[(2-methyl-acryloyl)oxy]ethyl] succinate (Mn=230, 0.46g) into dichloromethane, Add N,N'-dicyclohexylcarbodiimide (Mn=206, 0.41g) and triethylamine (0.5mL) and stir for 24 hours. Then it was washed with acid water and passed through the column to obtain the product Boc-HEMASN38. After detection, 1H-NMR10.238(s), 7.935(d), 7.35(d), 6.927(s), 5.89(s), 5.536(s), 5.409(s), 5.197(s), 4.262(d ), 4.135(d), 3.269(s), 3.011(d),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com