Medical adsorbent and method for producing same
A manufacturing method and adsorbent technology, applied in medical science, chemical instruments and methods, absorbent pads, etc., can solve the problems of reducing particle size, not necessarily sufficient adsorption performance, and large dosage, and achieve high effect and high adsorption capacity. Excellent selective adsorption and the effect of suppressing environmental burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
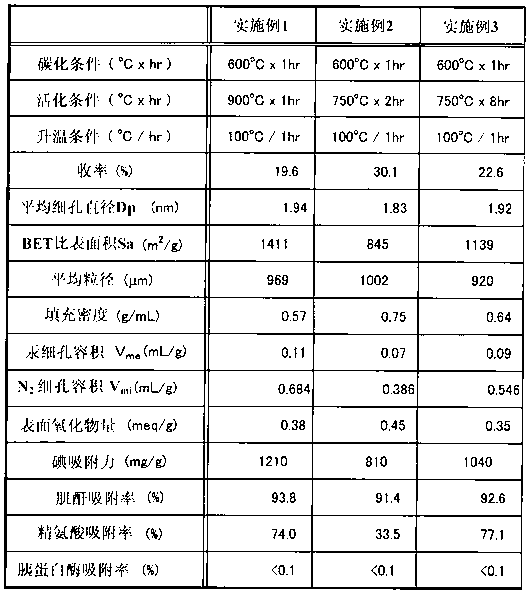
Embodiment 1)
[0062] 2 kg of dissolving pulp LNDP (manufactured by Nippon Paper Chemicals Co., Ltd.) with 90% by weight of α-cellulose per unit weight was immersed in a sodium hydroxide solution (18.5% concentration) at 55° C. for 15 minutes, and then pressed. The remaining sodium hydroxide component was removed to prepare alkali cellulose (AC) having a cellulose concentration of 33.5% by mass. The alkali cellulose was matured at 40°C for 7 hours, and 5 g of the alkali cellulose was reacted with 436 mL of carbon disulfide with a purity of 97% or higher for 70 minutes to obtain xanthate cellulose with a viscosity of 0.055 Pa·s (55 cP) at 40°C.
[0063] After the reaction was finished, about 13L of dilute sodium hydroxide solution was added to the cellulose xanthate, and stirred for 100 minutes to obtain viscose. After further defoaming, aging, and filtering processes, viscose with a cellulose concentration of 9.0% was prepared. Dilute the above-prepared viscose with distilled water to a vis...
Embodiment 2)
[0066] The activation temperature in Example 1 was set to 750° C., and the activation time was set to 2 hours, except that the granular activated carbon of Example 2 was obtained according to Example 1 (the yield was 30.1%).
Embodiment 3)
[0068] The activation temperature in Example 1 was set to 750° C., and the activation time was set to 8 hours, except that the granular activated carbon of Example 3 was obtained according to Example 1 (the yield was 22.6%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com