Method for preparing electronic-grade manganese sulfate by utilizing tungsten ore alkaline leaching slag
An alkali leaching, electronic-grade technology, applied in the direction of manganese sulfate, etc., can solve the problems of high cost and long impurity removal process, and achieve the effects of efficient recycling, simplified impurity removal process, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
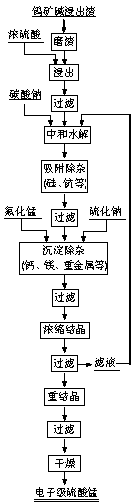
[0023] Grind tungsten ore alkali leaching slag to a particle size of 75 Next, take 200g, put it into the leaching tank, slowly add 240g of sulfuric acid with a concentration of 70%, turn on the stirrer, react and leaching at 70°C for 1.5h, and then filter. Heat the filtrate to 60°C, add sodium carbonate to adjust the pH value of the solution to 4, hydrolyze the iron and aluminum ions in the solution to generate corresponding hydroxide colloids, cool the solution to 30°C, and stir for 3 hours to fully absorb the hydroxide colloids in the solution silicon, scandium and other impurities, and then stand and filter. The filtrate was heated to 80°C, the pH value was controlled to 4.5, 26g of manganese fluoride was added, and the reaction was stirred for 0.5h to make the Ca in the solution 2+ , Mg 2+ Wait for the impurity ions to form insoluble fluoride precipitates, then add 12g of sodium sulfide, stir for 2.5 hours to make the heavy metal impurity ions in the solution form insol...
Embodiment 2
[0026] Grind tungsten ore alkali leaching slag to a particle size of 105 Next, take 200g, put it into the leaching tank, slowly add 185g of sulfuric acid with a concentration of 80%, turn on the stirrer, react and leaching at 80°C for 2h, and then filter. Heat the filtrate to 70°C, add sodium carbonate to adjust the pH value to 5, hydrolyze the iron and aluminum ions in the solution to generate corresponding hydroxide colloids, cool the solution to 40°C, and stir for 2.5 hours to fully absorb the hydroxide colloids in the solution silicon, scandium and other impurities, and then stand and filter. The filtrate was heated to 90°C, the pH value was controlled to be 5, 28g of manganese fluoride was added, and the reaction was stirred for 1h to make the Ca in the solution 2+ , Mg 2+ Wait for the impurity ions to form insoluble fluoride precipitates, then add 15g of sodium sulfide, stir for 2 hours to make the heavy metal impurity ions in the solution form insoluble sulfide preci...
Embodiment 3
[0029] Grind tungsten ore alkali leaching slag to a particle size of 150 Next, take 200g, put it into the leaching tank, slowly add 145g of sulfuric acid with a concentration of 90%, turn on the stirrer, react and leaching at 90°C for 1.5h and then filter. Heat the filtrate to 80°C, add sodium carbonate to adjust the pH value of the solution to 6, hydrolyze the iron and aluminum ions in the solution to generate corresponding hydroxide colloids, cool the solution to 50°C, and stir for 2 hours to fully absorb the hydroxide colloids in the solution silicon, scandium and other impurities, and then stand and filter. The filtrate was heated to 95°C, the pH value was controlled to 6.5, 25g of manganese fluoride was added, and the reaction was stirred for 1.5h to make the Ca in the solution 2+ , Mg 2+ Wait for the impurity ions to form insoluble fluoride precipitates, then add 18g of sodium sulfide, stir for 1.5 hours to make the heavy metal impurity ions in the solution form insol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com