High-sensitivity protein detection method
A protein detection and high-sensitivity technology, which is applied in the field of high-sensitivity protein detection, can solve the problems of increased magnetic bead background, high requirements for magnetic bead washing technology, and affecting sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
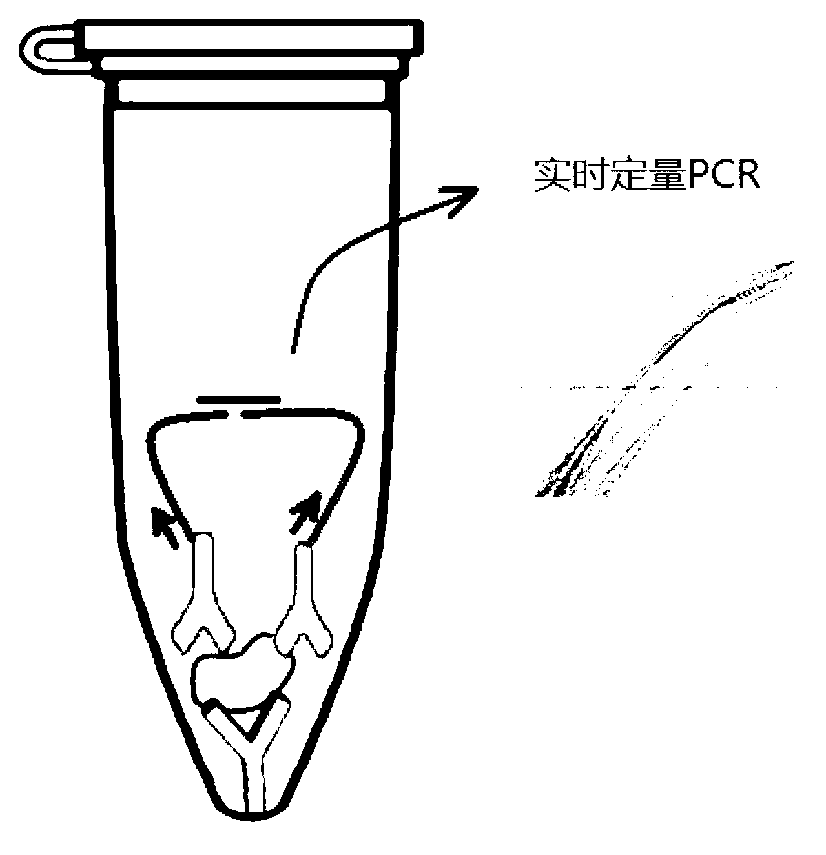
[0078] Embodiment 1. A high-sensitivity protein detection method, using the direct coating method of mode 1 to carry out solid-phase PLA on the tube wall, specifically the following steps:
[0079] 1) Add 50 ul of PAB240 antibody at a concentration of 0.1 ug / ml to each quantitative PCR tube, place it at 4°C for 12 hours, and wash with PBST buffer 3 times (each volume is 200 ul); the purpose of washing It is to wash away the antibody that is not bound to the tube wall.
[0080] 2) Add 200 ul of BSA solution with a concentration of 10g / L to each of the quantitative PCR tubes coated with antibodies above (that is, each quantitative PCR tube obtained in step 1), and discard the BSA solution after standing at 37°C for 2 hours , after drying at 25 ℃, put it at 4 ℃ for later use; the shelf life at 4 ℃ can reach 6-12 months.
[0081] 3) 100 microliters of two biotinylated single-stranded DNAs (probe DNA1 and probe DNA2) with a concentration of 100 nanomoles / liter, and carry out the f...
Embodiment 2
[0112] Embodiment 2. A high-sensitivity protein detection method, using the cross-linking agent immobilization method of mode 2 to carry out solid-phase PLA on the tube wall, specifically:
[0113] Insert the following prologue steps before step 1) of Example 1:
[0114] Add 50 ul / tube of cross-linking agent solution (glutaraldehyde solution with volume concentration of 1%) to the quantitative PCR tube, place it at 37°C for 5 hours, and wash it with deionized water for 3 times (200 ul for each time; The purpose is to remove the unreacted cross-linking agent in the PCR tube); get the quantitative PCR tube after pretreatment.
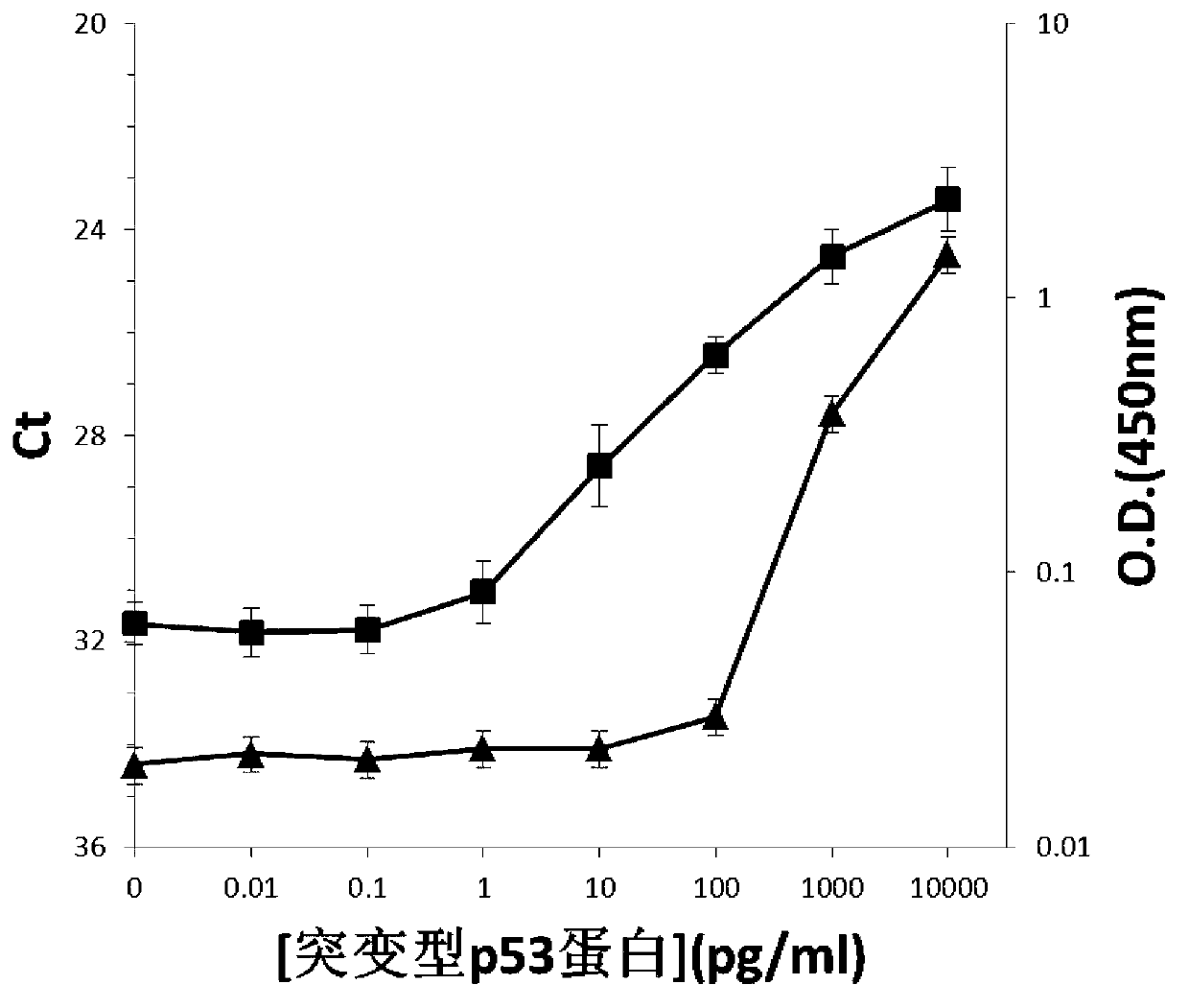
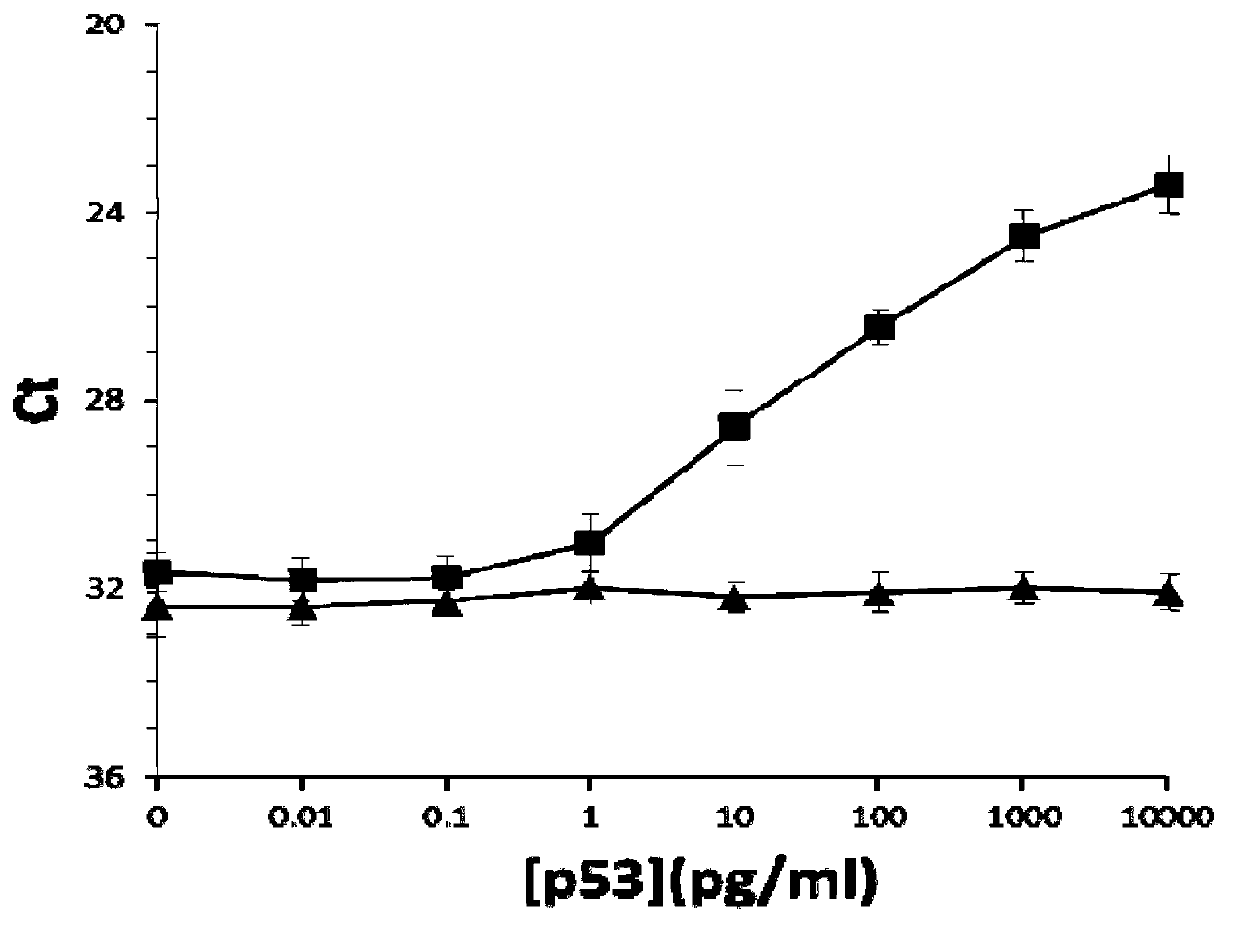
[0115]Then use this pre-treated quantitative PCR tube to replace the quantitative PCR tube in Step 1) of Example 1, and the rest are the same as in Example 1. At the same time, the pre-treated quantitative PCR tube was used instead of the quantitative PCR tube in Step 1) of Example 1, as in verification test 2 in Example 1, to detect the normal P53 prote...
Embodiment 3
[0125] Example 3. Detection of cardiac troponin cTnI
[0126] Cardiac troponin cTnI in human serum is closely related to myocardial infarction. We use cross-linking agent immobilization method of mode 2 with higher detection sensitivity to detect cardiac troponin cTnI by solid-phase PLA on the tube wall. That is, according to Example 2, 50 ul / tube of a cross-linking agent solution (glutaraldehyde solution with a volume concentration of 1%) was added to the quantitative PCR tube, and after standing at 37°C for 5 hours, it was washed 3 times with deionized water (each The amount used is 200 ul; the purpose of washing is to remove the unreacted cross-linking agent in the PCR tube); the quantitative PCR tube after pretreatment is obtained. Then use this pre-treated quantitative PCR tube to replace the quantitative PCR tube in step 1) of Example 1, but use cTnI monoclonal antibody (purchased from American R&D Company, catalog number: ab47003) to replace the p53 mutant monoclonal a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com