Preparation method of flucloxacillin sodium crystal form II
A technology of flucloxacillin sodium and crystal form, applied in the direction of organic chemistry, etc., can solve problems such as unstable solubility properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
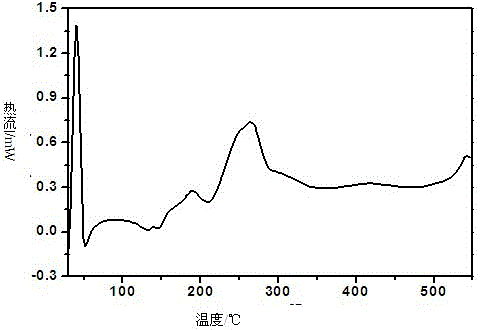
[0057] 1. Preparation of flucloxacillin sodium crystal form I:
[0058] Add 1.0 g of flucloxacillin sodium bulk drug and 17 mL of isopropanol to a 50 mL three-necked flask, stir magnetically, and stir at a speed of 20 r / min. After heating at 50-55°C for 0.5h, filter while hot, collect the filtrate, cool at room temperature for crystallization, collect crystals after precipitation, and dry at 50°C at a constant temperature. After drying for 12 hours, keep in a desiccator at room temperature.
[0059] 2. Confirmation of flucloxacillin sodium crystal form I:
[0060] 1. Ultraviolet spectrophotometry:
[0061] Phosphate buffer solution with pH=6.8 was used to prepare 1g / L flucloxacillin sodium standard and flucloxacillin sodium crystal form I solution, quartz cuvette, and phosphate buffer solution with pH=6.8 as blank reagent.
[0062] Operating Conditions:
[0063] Detector: UV-2450 Ultraviolet Spectrophotometer
[0064] As a result, the crystalline form I of flucloxacillin...
Embodiment 2
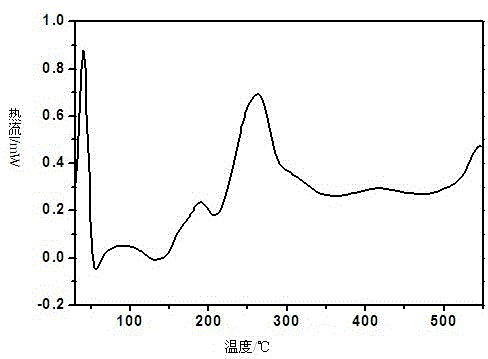
[0083] 1. Preparation of flucloxacillin sodium crystal form II:
[0084] Add 2.0 g of flucloxacillin sodium bulk drug and 20 mL of ethanol to a 50 mL three-necked flask, stir magnetically, and stir at a speed of 20 r / min. After heating for 0.5h under boiling conditions, filter while hot, slowly cool for crystallization, collect crystals after precipitation, and dry them at a constant temperature of 50°C, and store them in a desiccator at room temperature after drying for 12 hours.
[0085] 2. Confirmation of the crystal form II of flucloxacillin sodium:
[0086] 1. Ultraviolet spectrophotometry:
[0087] Phosphate buffer solution with pH=6.8 was used to prepare 1g / L flucloxacillin sodium standard and flucloxacillin sodium crystal form II solution, quartz cuvette, and phosphate buffer solution with pH=6.8 as blank reagent.
[0088] Operating Conditions:
[0089] Detector: UV-2450 Ultraviolet Spectrophotometer
[0090] As a result, the crystalline form II of flucloxaci...
Embodiment 3
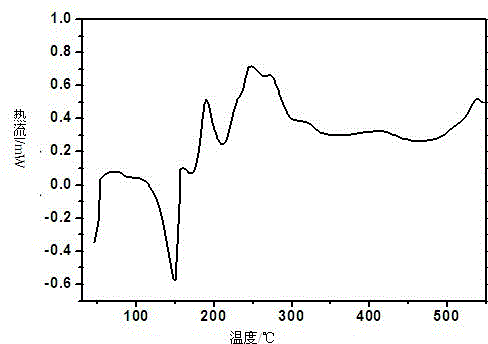
[0109] 1. Preparation of flucloxacillin sodium crystal form III:
[0110] Add 1.0 g of flucloxacillin sodium bulk drug and 13 mL of acetone into a 50 mL three-necked flask, stir with magnetic force at a stirring speed of 20 r / min. After heating under boiling conditions for 0.5h, filter while it is hot, cool and crystallize at a lower temperature (5-15°C), collect the crystals after precipitation and dry at a constant temperature of 50°C, dry for 12 hours and seal them in a desiccator at room temperature.
[0111] 2. Confirmation of flucloxacillin sodium crystal form III:
[0112] 1. UV spectrophotometry:
[0113] Use phosphate buffer solution with pH=6.8 to configure 1g / L flucloxacillin sodium standard and flucloxacillin sodium crystal form III solution, quartz cuvette, and phosphate buffer solution with pH=6.8 as blank reagent.
[0114] Operating conditions:
[0115] Detector: UV-2450 ultraviolet spectrophotometer
[0116] As a result, the crystal form III of flucloxa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com