Long-acting injectable preparation, preparation method and application thereof
A technology for long-acting injection preparations and injection preparations, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. and adverse effects on the stability of preparations, reducing the shelf life of drugs and drug safety, etc., to achieve the effects of fast administration, reducing economic burden, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
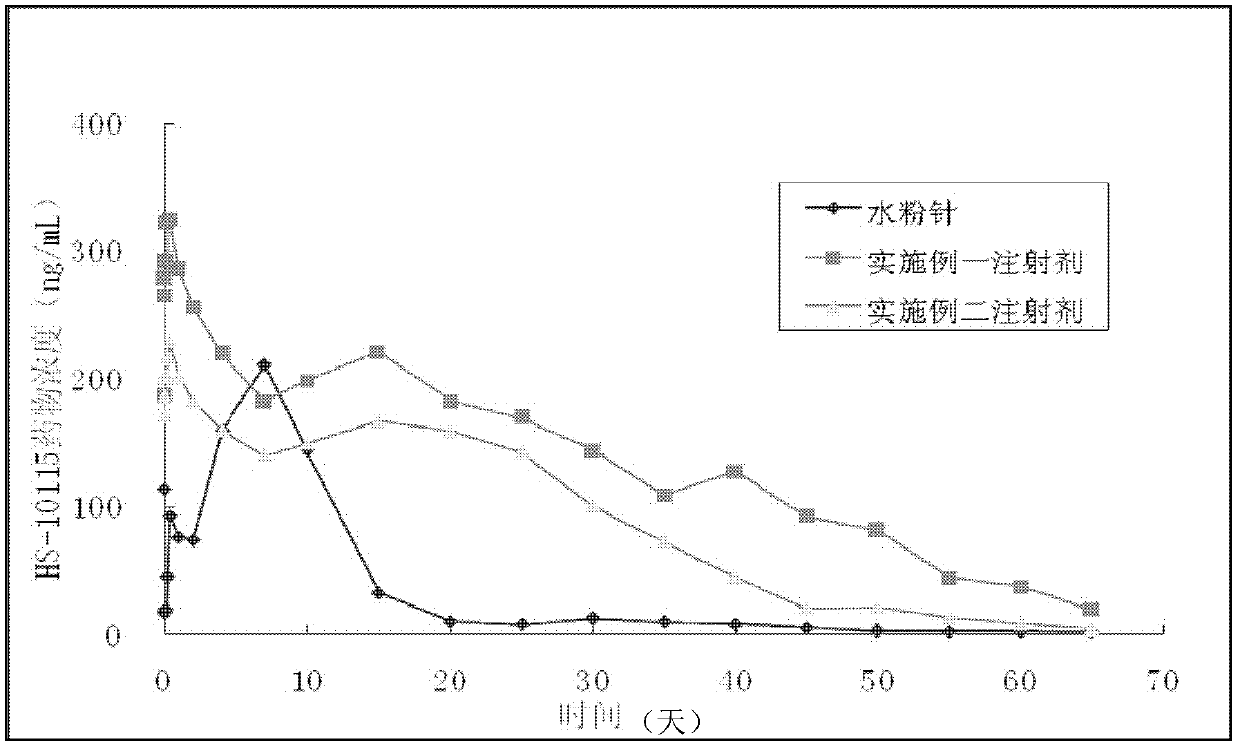
[0034] Embodiment 1 prepares aripiprazole injection (100mg / ml)
[0035] Mix 100 g of aripiprazole with 100 ml of absolute ethanol and 50 ml of benzyl alcohol and stir until completely dissolved. Add 200ml of benzyl benzoate, mix thoroughly, then slowly add 650ml of peanut oil under stirring to make the final solution. During preparation, attention should be paid to the order and speed of addition of each component, otherwise drug crystals will be precipitated. The solution needs to be blanketed with nitrogen, and then filtered through one or two 0.2 μm pore size filters to sterilize. The sterile filtrate is maintained under a nitrogen blanket while the solution is aseptically filled into cleansed and depyrogenated sterile primary containers such as vials or prefilled syringes. Appropriately add excess solution in the primary packaging container for easy access to the administered dose. Primary packaging containers are blanketed with sterile nitrogen and then aseptically sea...
Embodiment 2
[0036] Embodiment 2 prepares aripiprazole injection (100mg / ml)
[0037] Mix 100 g of aripiprazole with 100 ml of absolute ethanol and 100 ml of benzyl alcohol and stir until completely dissolved. Add 300ml of benzyl benzoate, mix well, then slowly add 500ml of a pre-mixed mixture of sesame oil and castor oil (250ml sesame oil and 250ml castor oil) under stirring to make the final solution. During preparation, attention should be paid to the order and speed of addition of each component, otherwise drug crystals will be precipitated. The solution needs to be blanketed with nitrogen, and then filtered through one or two 0.2 μm pore size filters to sterilize. The sterile filtrate is maintained under a nitrogen blanket while the solution is aseptically filled into cleansed and depyrogenated sterile primary containers such as vials or prefilled syringes. Appropriately add excess solution in the primary packaging container for easy access to the administered dose. Primary packagin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com