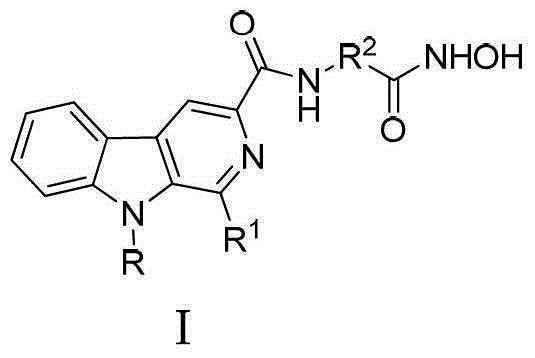
Beta-carboline derivative containing hydroximic acid as well as preparation method and medical application thereof
A kind of derivative, carboline technology, used in the preparation of malignant tumor, β-carboline derivatives and their pharmaceutically acceptable salts, Alzheimer's and Parkinson's disease medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
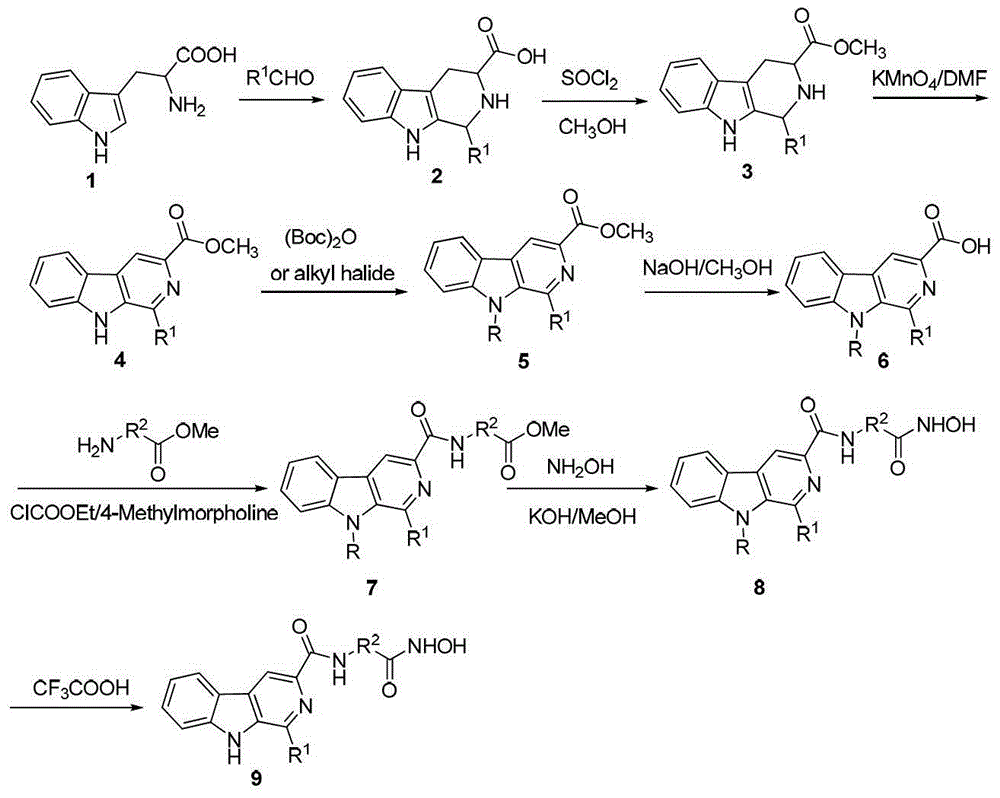
Examples
Embodiment 1
[0071] Embodiment 1N-(2-(hydroxylamino)-2-oxaethyl)-1-methyl-9-Boc-β-carboline-3-formamide (Ⅰ 1 ) preparation
[0072] 1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (2a)
[0073] Dissolve L-tryptophan (10.2g, 50mmol) in 20ml of 2.5mol / L H 2 SO 4 To the solution, 13.8ml of 40% acetaldehyde solution (115mmol) was added, and stirred at 60°C for 2h. After the reaction was completed, the reaction solution was adjusted to PH=5 with 2M HCl, and a large amount of white solid was precipitated, which was filtered by suction, washed with water, and dried in vacuo to obtain 9.8 g of white solid with a yield of 85.2%.
[0074] Methyl 1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylate (3a)
[0075] Dissolve 2a (11.5g, 50mmol) in 100ml of methanol, and slowly add 10.9ml of SOCl dropwise under low temperature and vigorous stirring at -5°C 2 (17.85g, 150mmol), heat and reflux for 2h after half an hour, pressurize and concentrate to remove the solvent after the reaction is c...
Embodiment 2
[0086] Embodiment 2N-(2-(hydroxylamino)-2-oxaethyl)-1-methyl-β-carboline-3-formamide (I 2 ) preparation
[0087] Will I 1 (0.1g, 0.25mmol) dissolved in CF 3 COOH / CH 2 Cl 2 (v / v=1:1, 10mL in total), stirred overnight at room temperature, after the reaction was complete, evaporated the solvent to obtain I 2 , yield 94.9%. ESI-MS(m / z):299[M+H] + .
Embodiment 3
[0088] Embodiment 3N-(4-(hydroxylamino)-4-oxabutyl)-1-methyl-9-Boc-β-carboline-3-carboxamide (I 3 ) preparation
[0089] Preparation of N-(4-(methoxy)-4-oxabutyl)-1-methyl-9-Boc-β-carboline-3-carboxamide (7b)
[0090] Referring to the preparation method of 7a, a yellow oil was obtained from 6a and γ-butyric acid methyl ester with a yield of 73.8%.
[0091] N-(4-(hydroxylamino)-4-oxabutyl)-1-methyl-9-Boc-β-carboline-3-carboxamide (I 3 ) preparation
[0092] Refer to I 1 The preparation method of , a yellow solid was obtained from 7b and hydroxylamine, and the yield was 66.1%. 1 H NMR (CDCl 3 ,300MHz):δ8.76(s,1H,H-4),δ8.69(m,1H,N H CH 2 ),8.27(m,1H,H-5),8.08(m,1H,H-8),7.60(m,H,H-6),7.28(m,1H,H-7),3.79(m, 2H, NCH 2 ),2.92(s,3H,CH 3 ),2.67(m,2H,CH 2 C=O),2.01(s,1H,OH),1.91(m,2H,CH 2 C H 2 CH 2 ),1.26(s,9H,-C(CH 3 ) 3 ).ESI-MS(m / z):427[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com