Method for producing unsaturated nitrile
A manufacturing method and unsaturated technology, applied in chemical instruments and methods, organic chemical methods, temperature control, etc., can solve the problem of composition deviation from the design value, and achieve the effect of maintaining yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0242] (Preparation of niobium mixture)
[0243] The niobium mixture was prepared as follows.
[0244] Mix 76.33kg of 80.2% by mass Nb in 500kg of water 2 o 5 of niobic acid and 29.02kg of oxalic acid dihydrate [H 2 C 2 o 4 2H 2 O]. The oxalic acid / niobium molar ratio of the feed was 5.0, and the niobium concentration of the feed was 0.532 (mol-Nb / Kg-liquid). By heating and stirring this liquid at 95° C. for 2 hours, a mixed liquid in which niobium was dissolved was obtained. The mixed solution was allowed to stand and ice-cooled, and the solid was filtered and separated by suction to obtain a uniform niobium mixed solution. According to the following analysis, the molar ratio of oxalic acid / niobium in this niobium mixed solution was 2.70.
[0245] Accurately weigh 10g of the niobium mixture into a crucible, dry at 95°C for one night, and heat treat at 600°C for 1 hour to obtain 0.7868g of Nb 2 o 5 . According to this result, the niobium concentration was 0.592 (mo...
Embodiment 2
[0261] A composite oxide catalyst was obtained by the same method as in Example 1.
[0262] (Ammoxidation of propane)
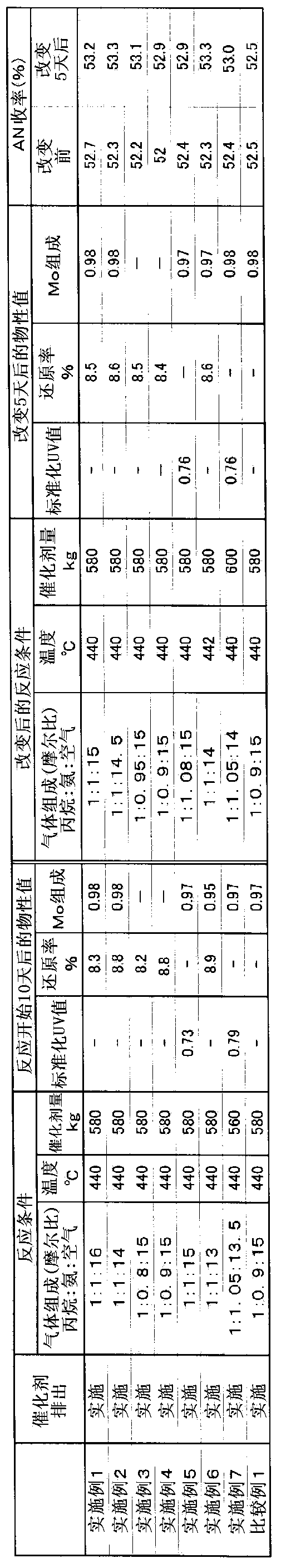
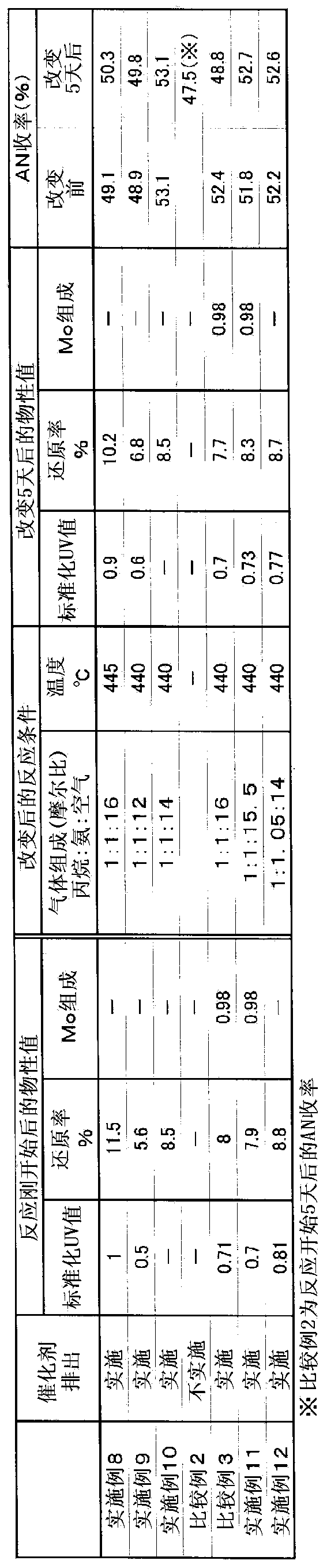
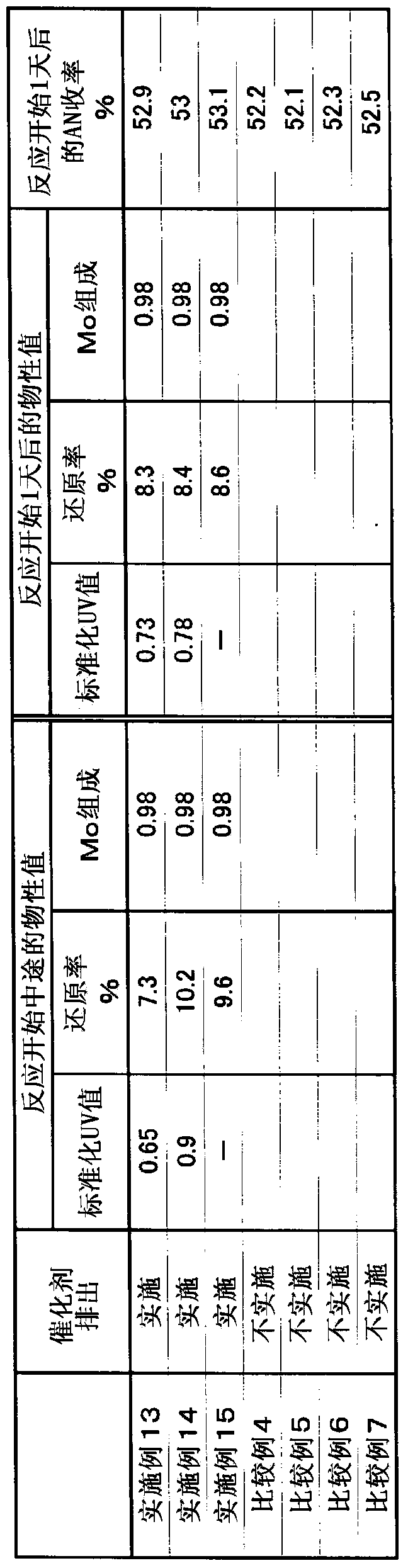
[0263] The reaction was performed in the same manner as in Example 1, except that gas was supplied at a molar ratio of propane:ammonia:air=1:1:14. Ten days after the start of the reaction, 500 g of the catalyst was discharged from the reactor, and the physical property values shown in Table 1 were measured. Table 1 shows the measurement results of various physical properties and the yield of AN at this time. Further, the molar ratio of air / propane introduced into the reactor was increased by 0.5, and the operation was continued. Five days after the conditions were changed, the catalyst was similarly discharged from the reactor, and the physical properties and yield were measured. The results are shown in Table 1.
Embodiment 3
[0265] A composite oxide catalyst was obtained by the same method as in Example 1.
[0266] (Ammoxidation of propane)
[0267] The reaction was performed in the same manner as in Example 1, except that gas was supplied at a molar ratio of propane:ammonia:air=1:0.8:15. Ten days after the start of the reaction, 500 g of the catalyst was discharged from the reactor, and the physical property values shown in Table 1 were measured. Table 1 shows the measurement results of various physical properties and the yield of AN at this time. Further, the molar ratio of ammonia / propane introduced into the reactor was increased by 0.15, and the operation was continued. Five days after the conditions were changed, the catalyst was similarly discharged from the reactor, and the physical properties and yield were measured. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com