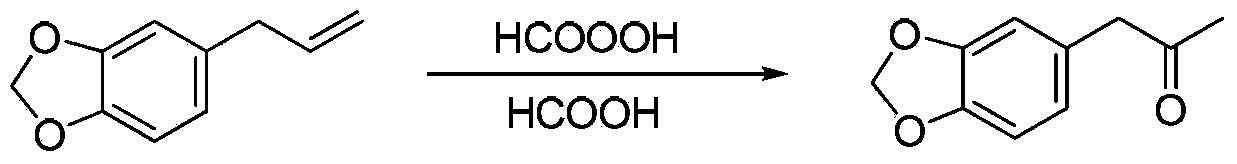A method for preparing 3,4-methylenedioxyphenyl-2-propanone using furanol by-product as raw material
A methylene dioxyphenyl and furan phenol technology, which is applied in directions such as organic chemistry, can solve the problems of scarcity of sassafras oil resources, unfriendly environment and high cost of raw materials, and achieves the effects of low production cost, simple process and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Under nitrogen protection, 30mL of water and 26.1g (0.15mol) of dibromomethane were refluxed. Mix 16.4g (0.10mol) 4-(2-methylallyl)-1,2-benzenediol and 10.0g (0.25mol) sodium hydroxide in 50mL water evenly, add after 2h, reflux for 2h, liquid Control the reaction in phase chromatography, cool after the reaction, add 100mL ethyl acetate for layering, wash the organic layer with 2% sodium hydroxide solution, dry, and distill to obtain 15.0g of light yellow liquid 5-(2-methallyl)benzene And[d][1,3]dioxole, content 98.3%, yield 83.4%. 1 H NMR (CDCl 3 , 300MHz) δ: 1.67(t, J=1.2Hz, 3H, CH 3 ), 3.23 (s, 2H, ArCH 2 ), 4.73(q, J=1.2Hz, 1H, C=CH), 4.79~4.80(q, J=1.2Hz, 1H, C=CH), 5.93(s, 2H, OCH 2 O), 6.63 ~ 6.75 (m, 3H, C 6 h 3 ).GC-MS(m / z):176(M + ), 151, 131, 103, 77.
Embodiment 2
[0034] Under nitrogen protection, 30mL of water and 17.4g (0.1mol) of dibromomethane were refluxed. Mix 16.4g (0.10mol) 4-(2-methylallyl)-1,2-benzenediol and 8.8g (0.22mol) sodium hydroxide in 50mL water evenly, add after 2h, reflux for 2h, liquid Control the reaction in phase chromatography, cool after the reaction, add 100mL of ethyl acetate for layering, wash the organic layer with 2% sodium hydroxide solution, dry, and distill to obtain 15.5g of light yellow liquid 5-(2-methallyl)benzene And[d][1,3]dioxole, content 97.9%, yield 85.7%.
Embodiment 3
[0036]Under nitrogen protection, 30mL of water and 34.8g (0.20mol) of dibromomethane were refluxed. Mix 16.4g (0.10mol) 4-(2-methylallyl)-1,2-benzenediol and 12.0g (0.30mol) sodium hydroxide in 50mL water evenly, add after 2h, reflux for 2h, liquid Control the reaction in phase chromatography, the reaction is complete; cool, add 100mL ethyl acetate to separate layers, wash the organic layer with 2% sodium hydroxide solution, dry, and distill to obtain 14.7g of light yellow liquid 5-(2-methylallyl) Benzo[d][1,3]dioxole, content 99.0%, yield 82.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com