Freeze-drying protective agent for vaccines
A technology of freeze-drying protective agent and vaccine, which is applied to medical preparations with non-active ingredients, non-effective ingredients of polymer compounds, and pharmaceutical formulas, etc. It can solve problems such as immune failure, poor protective performance, and discounted potency, and achieve operational Simplicity, low cost, and the effect of extending shelf life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
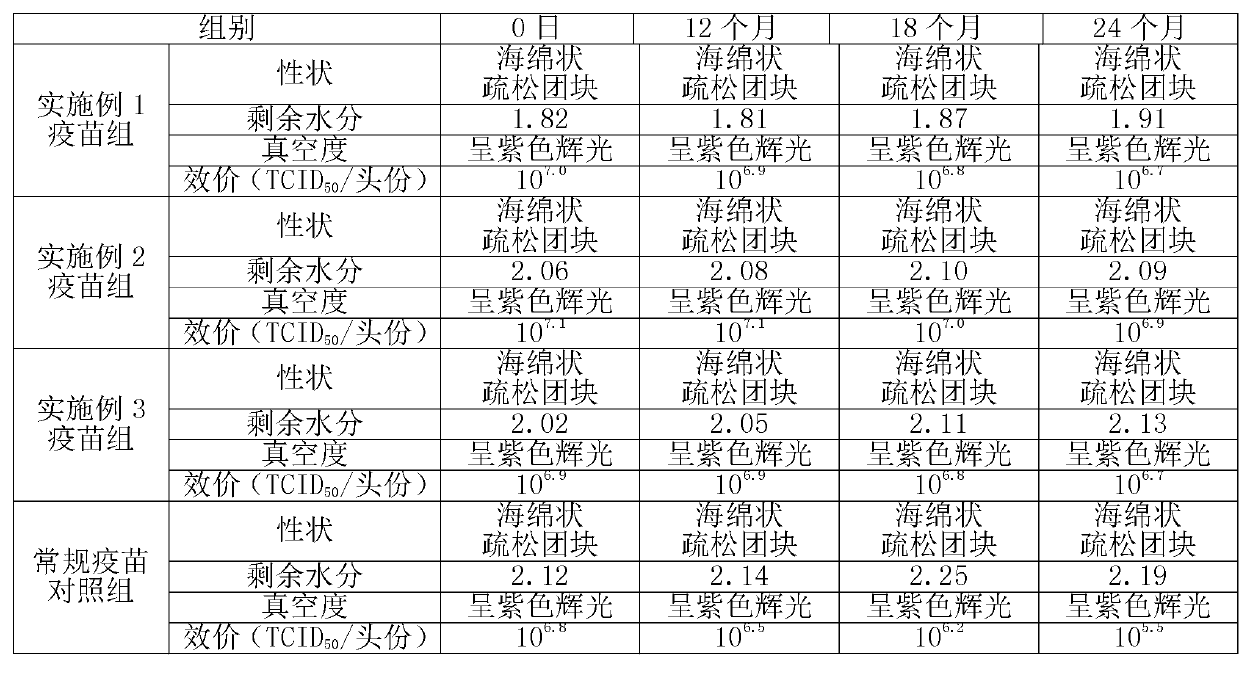
Image
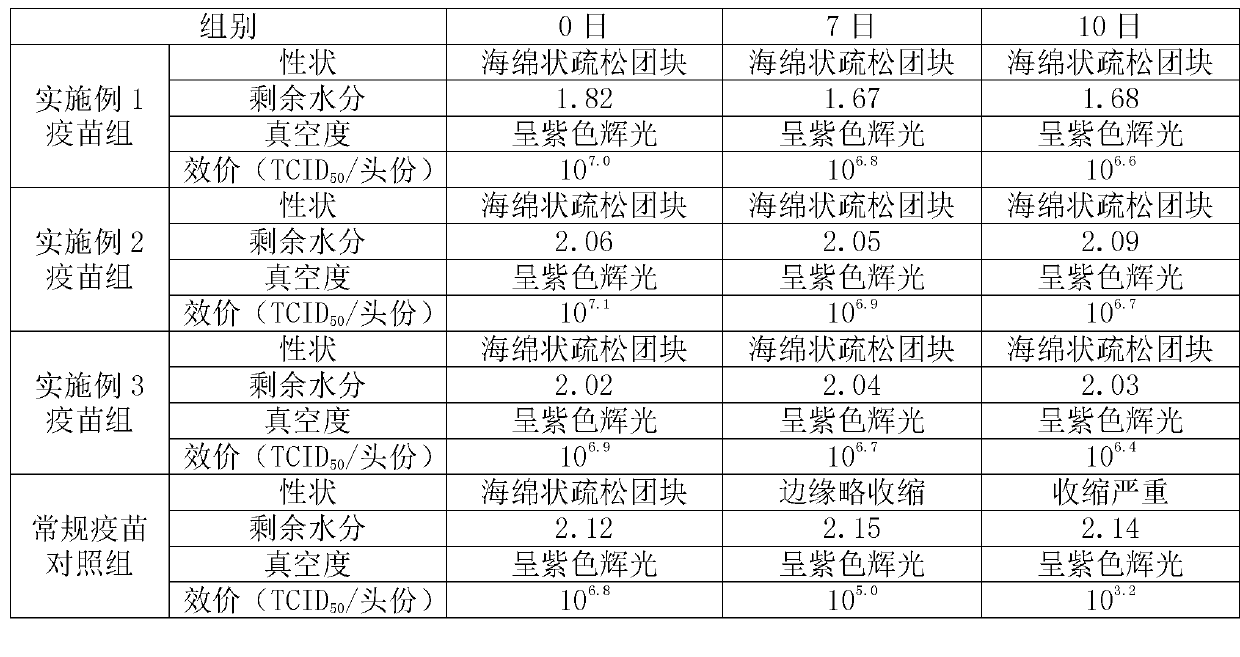
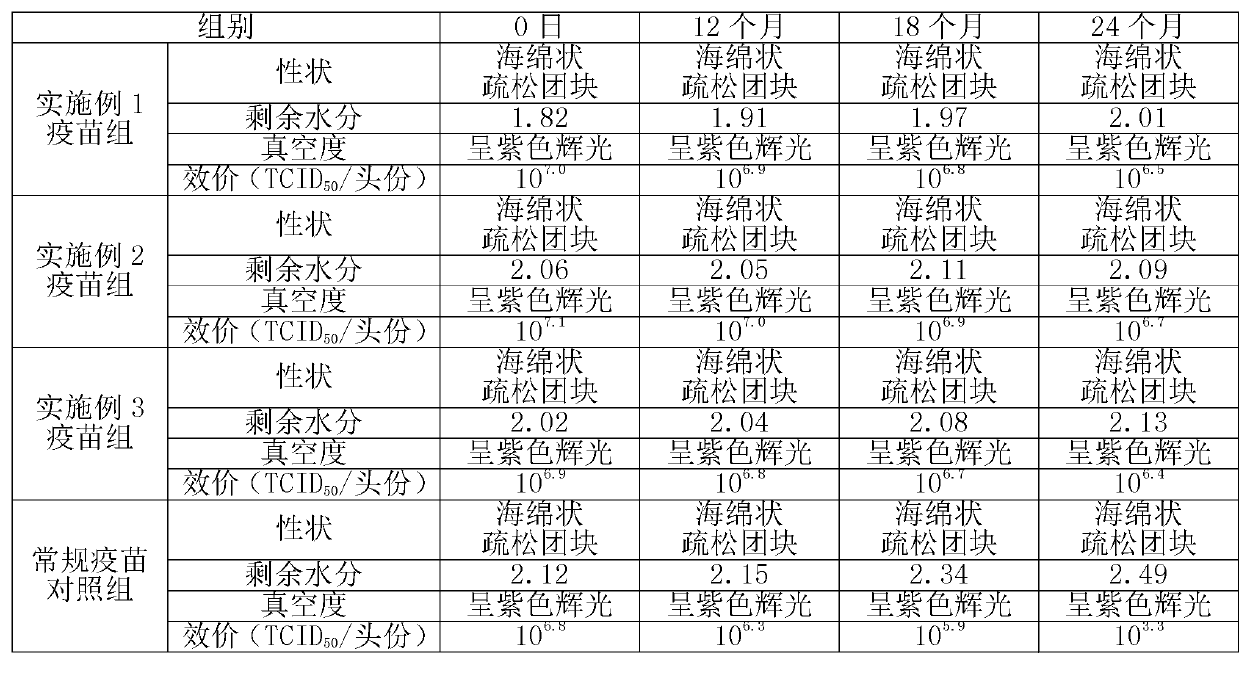
Examples
Embodiment 1
[0022] Formula and preparation method of freeze-dried freeze-dried protective agent: divided into two parts, A liquid and B liquid.
[0023] 1) Preparation of liquid A: Take 3 g of NZ amine, 0.375 g of monopotassium glutamic acid salt, 25 g of sucrose, and 1.875 g of hydrolyzed milk protein. Sterilize by membrane filtration and store at 2-8°C for later use.
[0024] 2) Preparation of liquid B: take 20g of hydrolyzed gelatin, fully dissolve it with 100ml of water for injection at 70-80°C, autoclave at 121°C and 15 pounds for 30 minutes, and store at 37°C after sterilization.
[0025] 3) Mix liquid A and liquid B at a ratio of 4:1, and mix well to obtain a freeze-dried freeze-dried protective agent. The mass and volume percentage concentrations of each component are as follows: 2.4% NZ-amine, 0.3% glutamine Monopotassium acid salt, 20% sucrose, 1.5% hydrolyzed milk protein, 4% hydrolyzed gelatin.
[0026] Preparation of vaccines using the lyoprotectant of the present invention...
Embodiment 2
[0033] 1. Formula and preparation method of freeze-dried freeze-dried protective agent: divided into two parts, A solution and B solution.
[0034] 1) Preparation of liquid A: NZ-amine 4wt%, glutamic acid monopotassium salt 0.5 wt%, sucrose 25 wt%, hydrolyzed milk protein 2.5 wt% (wt means weight to volume ratio g / ml); add the above ingredients in order Put it into water for injection, fully dissolve it, shake it well, filter it with a 0.22μm filter membrane, and store it at 2-8°C for later use.
[0035] 2) Preparation of liquid B: hydrolyzed gelatin 6 wt%, fully dissolved in water for injection at 70-80 °C, 121 °C, 15 lbs high pressure for 30 minutes, sterilized and stored at 37 °C.
[0036] 3) Mix liquid A and liquid B at a ratio of 1:1, mix well and then use it as a freeze-drying protective agent.
[0037] 2. Vaccine preparation
[0038] 1) After the porcine Japanese encephalitis virus is cultured, carry out sterility test and virus content determination, mix the qualifie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com