Preparation method of lithium iron phosphate
A lithium iron phosphate and lithium source technology, applied in chemical instruments and methods, phosphorus compounds, nanotechnology for materials and surface science, etc., can solve the problem of slow diffusion of lithium ions, poor conductivity of lithium iron phosphate, and increased process complexity issues such as safety and safety risks, and achieve the effects of large-scale commercial production, good electrochemical performance, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
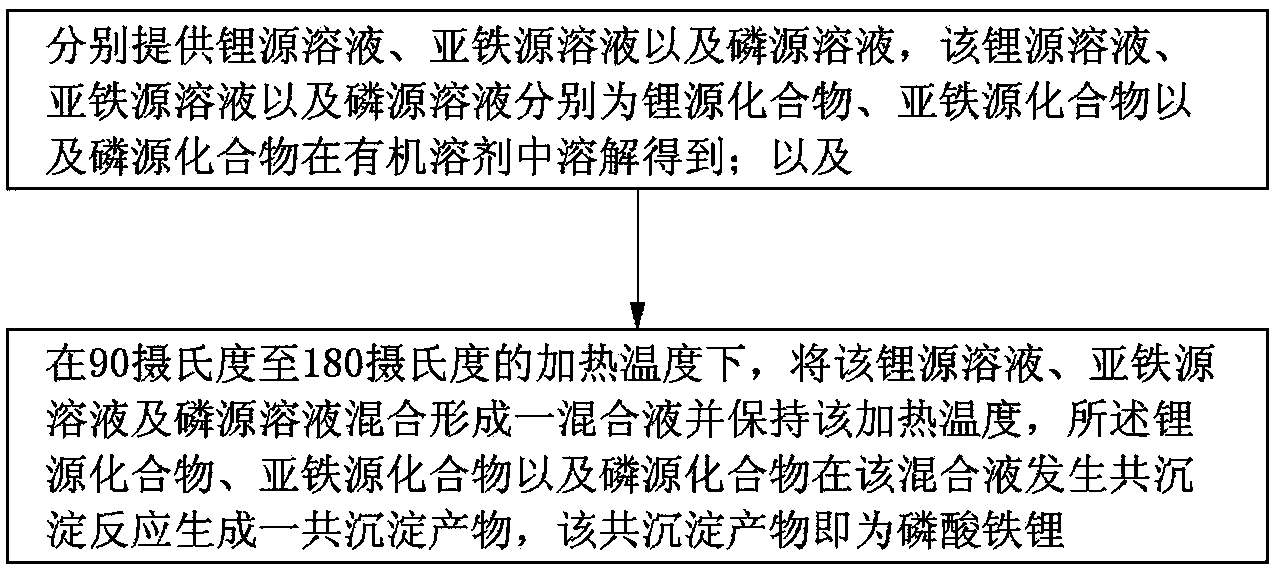
[0014] see figure 1 , the embodiment of the present invention provides a preparation method of lithium iron phosphate, which comprises the following steps:
[0015] S1, providing lithium source solution, ferrous source solution and phosphorus source solution respectively, the lithium source solution, ferrous source solution and phosphorus source solution are respectively obtained by dissolving lithium source compound, ferrous source compound and phosphorus source compound in an organic solvent ;as well as
[0016] S2, at a heating temperature of 90 degrees Celsius to 180 degrees Celsius, mix the lithium source solution, ferrous source solution and phosphorus source solution to form a mixed solution and maintain the heating temperature, the lithium source compound, ferrous source compound and phosphorus The source compound undergoes coprecipitation reaction in the mixed solution to generate a coprecipitation product, and the coprecipitation product is lithium iron phosphate. ...
Embodiment 1
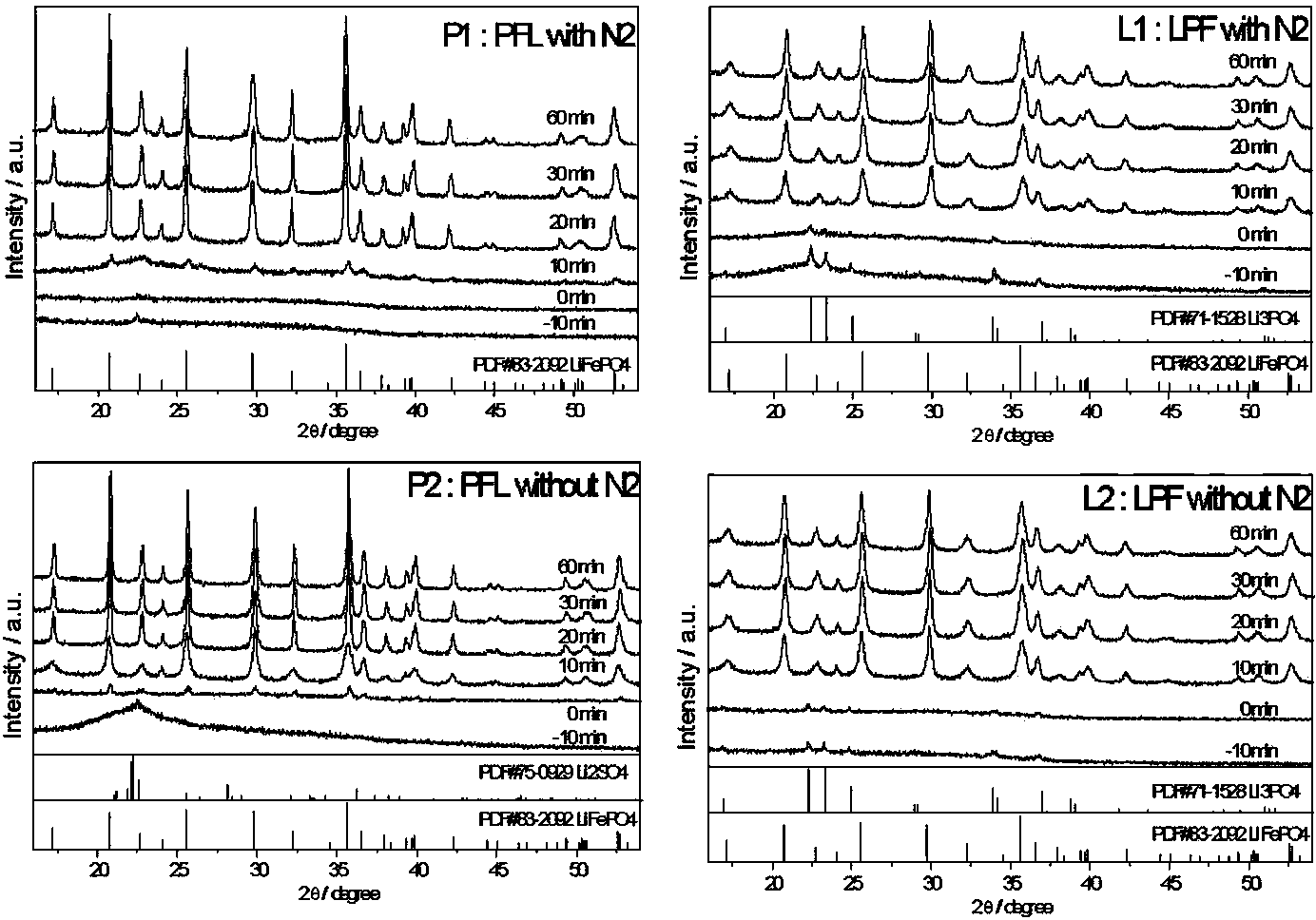
[0041] In this embodiment, the lithium source compound is lithium hydroxide (LiOH·H 2 O), the ferrous source compound is ferrous sulfate (FeSO 4 ·7H 2 O), the phosphorus source compound is phosphoric acid (H 3 PO 4 ), wherein lithium: iron: the molar ratio of phosphorus is 3:1:1. The organic solvent is ethylene glycol. The reactor uses a N 2 Protected airtight container, the heating method is silicone oil bath heating. First, under the condition of strong stirring, 0.03mol of H 3 PO 4 and FeSO 4 ·7H 2 O was dissolved in 100ml of ethylene glycol to form the first solution, and then the first solution was slowly added to 200ml of LiOH·H 2 O in ethylene glycol solution to form a mixture. The mixed solution was coprecipitated under heating at 180 degrees Celsius for 60 minutes to form coprecipitates. The co-precipitation reaction is carried out under normal pressure. The airtight container was naturally cooled to room temperature, and the co-precipitated product was c...
Embodiment 2
[0043] The basic conditions of this example are the same as those of Example 1, except that the co-precipitation reaction is carried out in an air environment, and the obtained product is marked as P2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com