Method of preparing calcium fluoride
A calcium fluoride and calcium salt technology, applied in the direction of calcium/strontium/barium fluoride, calcium/strontium/barium halide, etc., can solve the problems of low production rate, uneconomical, low purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] In order to better understand the features of the present invention, a preferred embodiment is listed below and described in conjunction with the drawings, wherein:
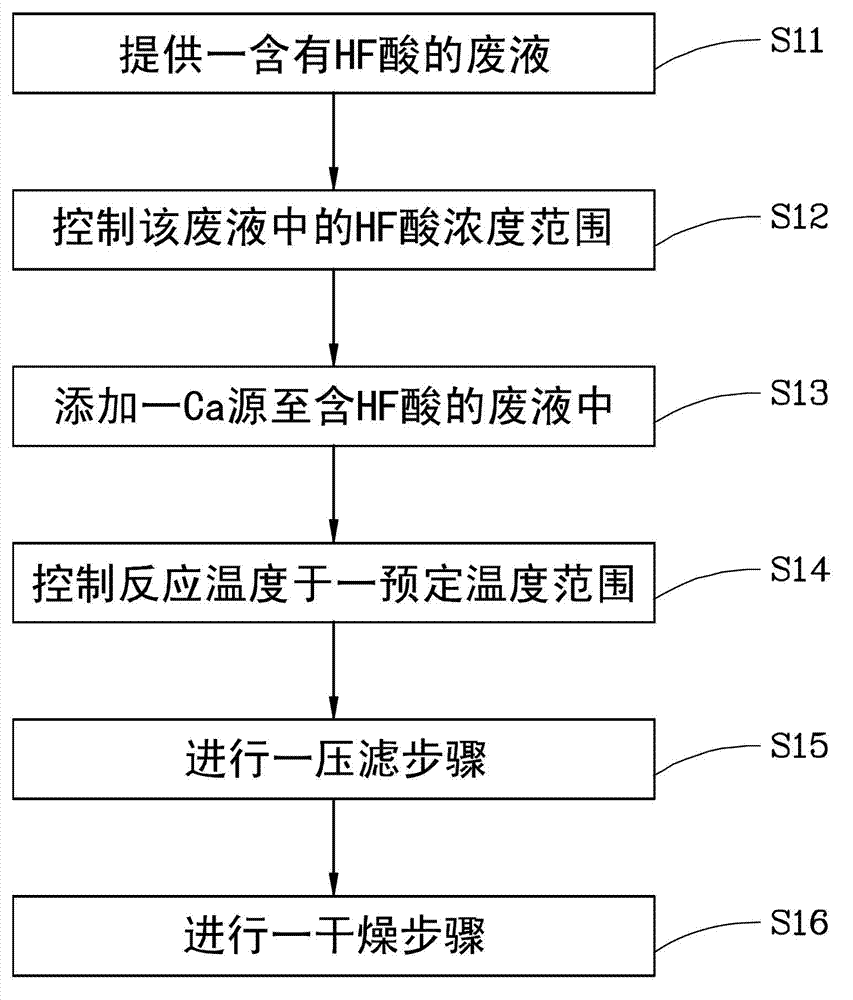
[0014] figure 1 It is a flowchart of a method for preparing calcium fluoride in a preferred embodiment of the present invention; and
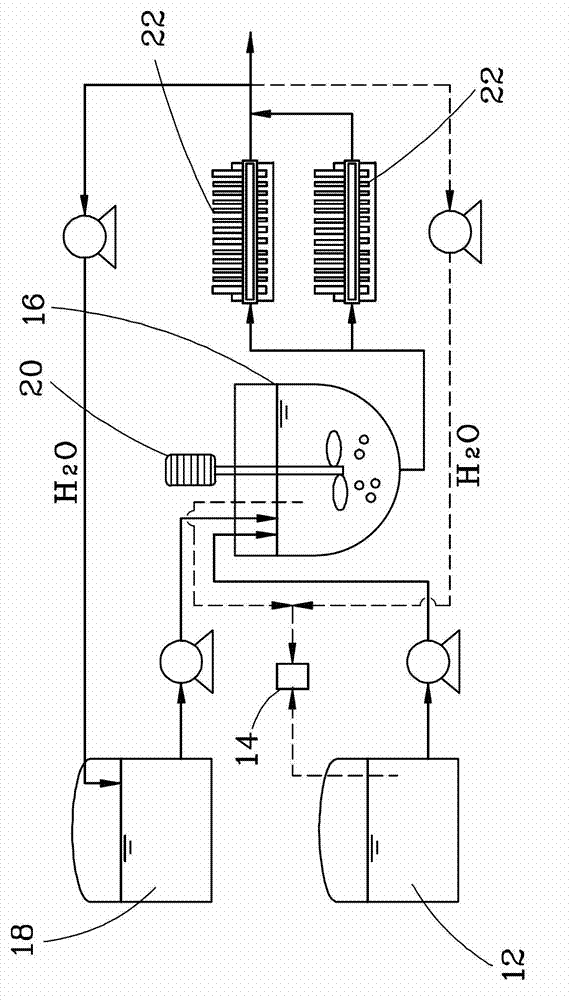
[0015] figure 2 It is a process schematic diagram of a calcium fluoride preparation method in a preferred embodiment of the present invention.
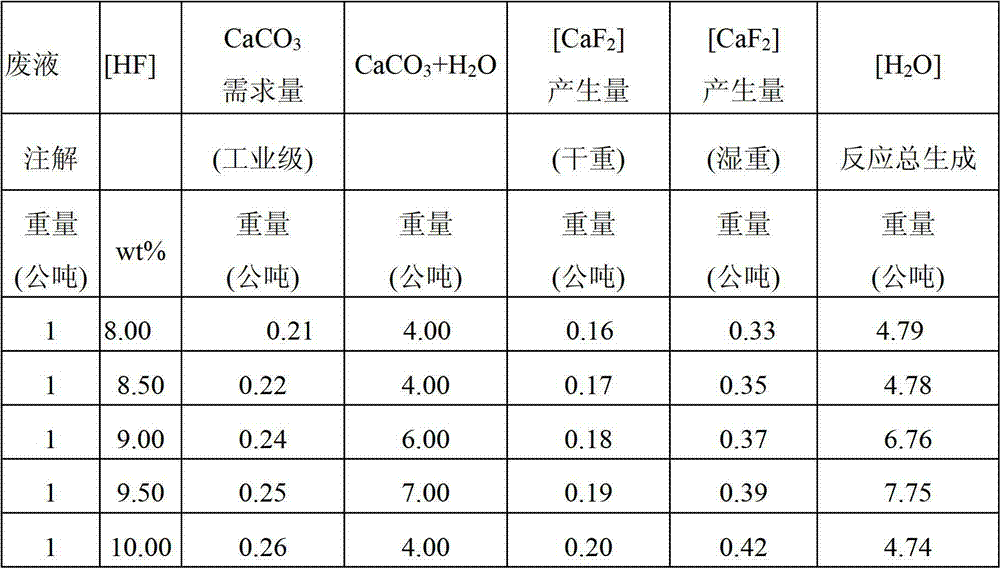
[0016] The waste liquids formed by different industries and different processes usually contain different concentrations of HF acid, the concentration can range from 3wt% to 49wt%, and some of these waste liquids may also contain impurities such as nitric acid and / or sulfuric acid, or even Fluorosilicide. The present invention is to provide a method for preparing calcium fluoride, which has simple manufacturing process, low cost, and CaF 2 The preparation rate is high, and the prepared CaF 2 It has extremely high and stable p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com