Preparation method of ofloxacin
A technology of ofloxacin and compounds, applied in the field of preparation of ofloxacin, can solve the problems of reduced yield, reduced content of final products, long chemical synthesis routes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
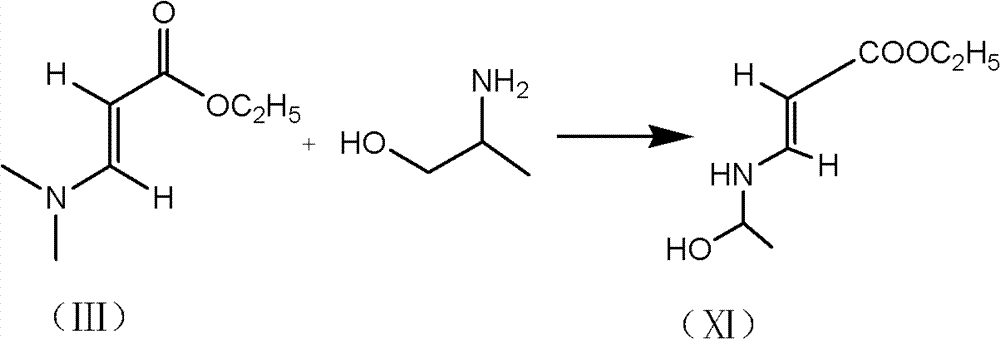
[0024] 1) Preparation of formula (V) compound
[0025] 300g of toluene, 35.5g of formula (III), and 17g of L-aminopropanol were successively put into a 500ml three-necked bottle, stirred and heated to 20°C, and timed to keep warm. After 8 hours of heat preservation, 8.5g of liquid ammonia and 60g of trimethylchlorosilane were added, and the temperature was kept at 50°C for 4 hours. It can be directly used in the next step without separation.
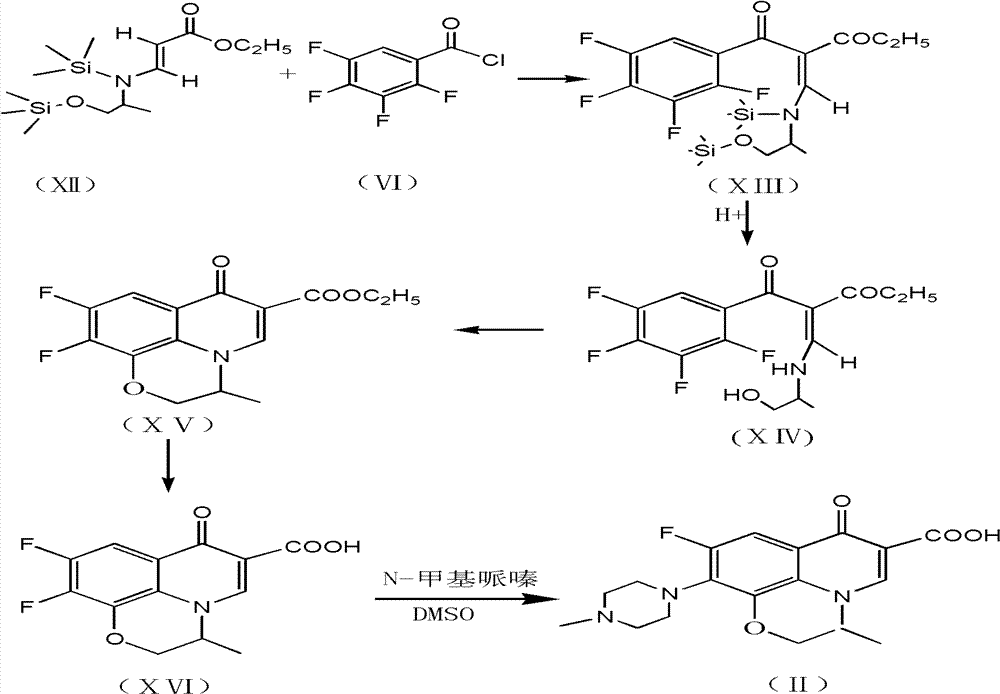
[0026] 2) preparation of formula (VIII) compound
[0027] The temperature of the toluene solution of formula (V) in the previous step is raised to 50°C, and it is stirred and dropped into formula (VI) for 3-4 hours. The formula (VII) is prepared by keeping the temperature at 50-100°C for 4 hours after dropping. Add 200ml of water, add hydrochloric acid to adjust the pH to <7.0, separate the water layer, recover toluene under reduced pressure, keep the temperature <90°C, and vacuum <-.09MPa. Stop when no toluene flows out, and the res...
Embodiment 2
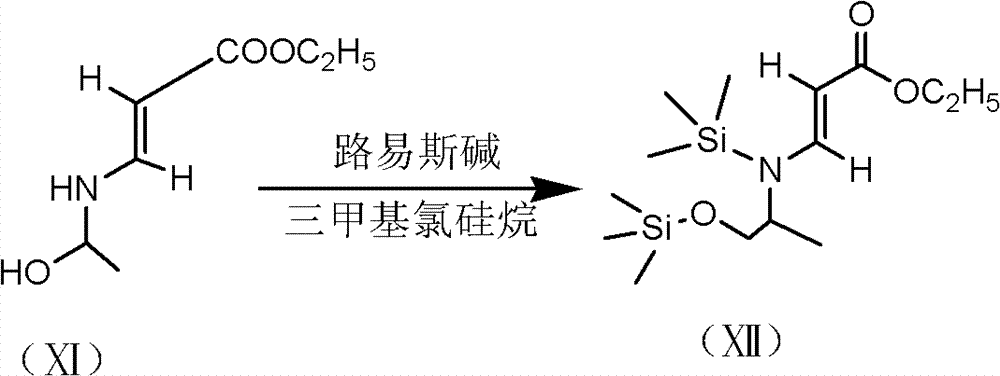
[0037] 1) Preparation of formula (XII) compound
[0038] 300g of toluene, 35.5g of formula (III), 17g of D, L-aminopropanol were put into a 500ml three-necked bottle in sequence, stirred and heated to 40°C, and timed to keep warm. After 6 hours of heat preservation, 75g of triethylamine and 60g of trimethylchlorosilane were added, and the temperature was kept at 50°C for 4 hours. It can be directly used in the next step without separation.
[0039] 2) Preparation of formula (XIV) compound
[0040] The temperature of the toluene solution of formula (XII) in the previous step is raised to 50°C, and it is stirred and dropped into formula (VI) for 3-4 hours. The formula (XIII) was prepared by keeping the temperature at 30-60°C for 4h after dropping. Add 200ml of water, add hydrochloric acid to adjust the pH to 7.0, separate the water layer, recover toluene under reduced pressure, keep the temperature <90°C, and vacuum <-.09MPa. Stop when no toluene flows out, and the residue i...
Embodiment 3
[0050] 1) Preparation of formula (V) compound
[0051] 300g of toluene, 35.5g of formula (III), and 17g of L-aminopropanol were successively put into a 500ml three-necked bottle, stirred and heated to 50°C, and timed to keep warm. After 4 hours of heat preservation, 20 g of ether, 15 g of ethanol, 25 g of ethanethiol, and 60 g of trimethylchlorosilane were added, and the mixture was kept at 50° C. for 4 hours. It can be directly used in the next step without separation.
[0052] 2) preparation of formula (VIII) compound
[0053] The temperature of the toluene solution of formula (V) in the previous step is raised to 50°C, and it is stirred and dropped into formula (VI) for 3-4 hours. The formula (VII) is prepared by keeping the temperature at 50-100°C for 4 hours after dropping. Add 200ml of water, add hydrochloric acid to adjust the pH to <7.0, separate the water layer, recover toluene under reduced pressure, keep the temperature <90°C, and vacuum <-.09MPa. Stop when no t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com